Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Acta Odontológica Latinoamericana
versão On-line ISSN 1852-4834
Acta odontol. latinoam. vol.28 no.3 Buenos Aires dez. 2015
ARTÍCULOS ORIGINALES
Impairment of bony crypt development associated with hexavalent chromium exposure during tooth eruption
Luciana M. Sánchez, Marianela Lewicki, Romina C. De Lucca, Ángela M. Ubios
Department of Histology and Embryology, School of Dentistry, University of Buenos Aires. Buenos Aires, Argentina
CORRESPONDENCE Luciana M. Sanchez Marcelo T. De Alvear 2142, 1° “A”. CP 1122AAH. Buenos Aires, Argentina. e-mail: lucianamsanchez@hotmail.com
ABSTRACT
Improperly treated hexavalent chromium-containing industrial wastes contaminate drinking water, potentially affecting children taking breast milk or baby bottles prepared with infant formula. Thus, the aim of the present work was to determine the effect of this toxic on bone activity in the developing alveolus during tooth eruption of suckling Wistar rats intoxicated with potassium dichromate. Experimental animals received a daily dose of 12.5mg/kg body weight of potassium dichromate by gavage for 10 days; controls received an equivalent volume of saline solution. Histologic and histomorphometric studies of the mandible were performed. The data were statistically analyzed using Student’s t test; statistical significance was set at a value of p <0.05. Experimental animals exhibited delayed tooth eruption, decreased periodontal width and bone volume, a lower percentage of bone formation surfaces, and higher percentage of quiescent surfaces (p<0.05) compared to controls. The delay in tooth eruption observed after exposure to hexavalent chromium is the result of a lower rate of bone remodeling in the developing alveolus. The obtained results show the importance of controlling toxic substances in drinking water, since their effects may alter the growth and development of subjects who were exposed during early infancy.
Key words: Tooth eruption; Hexavalent chromium; Bone remodeling; Drinking water.
RESUMEN
Alteración del desarrollo de la canastilla ósea asociada a la exposición de cromo hexavalente durante la erupción dentaria
Desechos industriales que contienen cromo hexavalente inade - cua damente tratados contaminan el agua de consumo pudiendo afectar a los ninos por via de la leche materna o de la preparacion de mamaderas. Por lo tanto, el objetivo del presente trabajo fue determinar el efecto de este toxico en la actividad del hueso en el alveolo en desarrollo durante la erupcion dentaria de ratas Wistar lactantes expuestas a dicromato de potasio. Los animales experimentales recibieron una dosis diaria de 12,5 mg / kg de peso corporal de dicromato de potasio por alimentacion forzada durante 10 dias; mientras que los controles, un volumen equivalente de solucion salina. Se llevaron a cabo estudios histologicos e histomorfometricos de la mandibula. Los datos fueron analizados estadisticamente utilizando la prueba t de Student; estableciendose un valor de p<0,05 como esta - disticamente significativo. Los animales expuestos a cromo hexavalente mostraron retraso en la erupcion dentaria, menor espacio periodontal y volumen oseo; encontrandose disminuidas las superficies en formacion y en reabsorcion oseas y aumentadas las superficies en reposo (p <0,05) en comparacion con los controles. El retraso en la erupcion dentaria observado luego de la exposicion a cromo hexavalente es el resultado de una menor remodelacion osea en el alveolo en desarrollo. Los resultados obtenidos muestran la importancia del control de sustancias toxicas en el agua potable, ya que sus efectos pueden alterar el crecimiento y el desarrollo de los individuos que fueron expuestos durante la infancia temprana.
Palabras clave: Erupcion dentaria; Cromo hexavalente; Remodelacion osea; Agua de consumo.
INTRODUCTION
Hexavalent chromium compounds produced by the chemical industry are used for the manufacture of dyes and pigments, leather tanning, and wood preserving. Wastes from electroplating, petrochemical industry, leather tanning, and textile industry can be released into the air or the soil, or be discharged into waterways, contaminating drinking water 1. The general population can be exposed to hexavalent chromium directly through skin contact, by inhaling air, or by drinking or eating foods contaminated with chromium 2,3. After entering the cell, Cr VI is reduced to Cr III, resulting in the formation of reactive intermediates which contribute to the cytotoxicity, genotoxicity, and carcinogenicity 4. In the year 2010 the US Environmental Protection Agency (EPA) 5 established that the maximum allowable concentration of total chromium which includes all forms of chromium including chromium-6 in drinking water should not exceed 0.1mg/l. or 100 parts per billion (ppb). Nevertheless, these recommendations are not met in some countries in America, Europe and Asia 6-11. There are studies in the literature associating exposure to hexavalent chromium and risk of bone damage 12,13. Sankaramanivel et al 14 reported that Cr VI has been found to enter the inorganic bone matrix of vertebrae, femur and calvaria of adults male rats, altering the tissue and interfering with bone formation and resorption, thus, leading to altered bone turnover.
According to the Agency for Toxic Substances and Disease Registry 15, babies could be exposed to high environmental levels of chromium through inhalation and consumption of contaminated foods -including breast milk- and water -used to prepare baby formula. Very few studies have investigated the effects of chromium exposure on children. However, it is likely that children would have the same health effects as adults. Soudani et al 16 found that exposure of rat dams to potassium dichromate before and after delivery affected growth and decreased bone mineral content of their progeny and De Lucca et al.17 demonstrated a decrease in body growth of suckling rats receiving potassium dichromate solutions. The association between other toxic substances and bone alterations is well documented: iron decreases bone formation and inhibits endochondral ossification 18, lead replaces the calcium in the hydroxyapatite crystals and also impairs body growth 19 and uranium affects bone remodeling, decreasing mandibular growth and delaying tooth eruption 20,21.
Tooth eruption is a highly dynamic biological process, in which bone tissue plays a crucial role. Little is known about bone remodeling in the walls of the alveolus as the tooth drifts during tooth eruption. Studies in rat molars, which, like human teeth, are teeth of limited eruption, have shown that bone resorption and formation are essential during the intraosseous and mucosal penetration stages of tooth eruption, when the walls of the dental alveolus develop 22,23. In their 2012 study in Jaipur, India, Tiwari et al 24 demonstrated the presence of hexavalent chromium in the blood of children working in gem polishing industries. It is of note that the studied children were at the age when the permanent second molars erupt (10-12 years). Although De Lucca et al 17 demonstrated that the exposure of suckling rats to hexavalent chromium resulted in decreased body and mandibular growth and delayed tooth eruption; it remains to be clarified whether these observations are the result of an alteration in bone remodeling.
Thus, the aim of the present work was to determine the effect of hexavalent chromium exposure on the developing alveolus during tooth eruption in suckling Wistar rats intoxicated with potassium dichromate.
MATERIAL AND METHODS
Sixteen 4-day-old suckling Wistar rats were assigned to one of two groups: an experimental and a control group. Under topical anesthesia [Xylocaine (XilocainaR, Astra Zeneca Argentina)], experimental animals received 12.5mg/kg body weight of potassium dichromate (Biopack, Argentina) daily by gavage through a flexible PVC tube. Control animals received an equivalent dose of saline solution under the same conditions as experimental pups. The litters were adjusted to 8 pups per dam and were housed with their mother in individual cages with wood-chip bedding, and kept on a controlled light-dark cycle (lights on at 7 am and off at 7 pm) and under constant humidity (40-70%). The mothers were fed a solid diet and water at libitum. After each procedure, the pups were returned to the cage with their mother. All the pups were euthanized on day 15 of the experiment and the mandibles were resected. The hemi-mandibles were fixed in 4% buffered formalin for 48 hours, decalcified in 10% EDTA pH 7, and embedded in paraffin. Buccal-lingual sections of the hemi-mandibles at the level of the mesial root of the first lower molar were obtained and stained with hematoxylin-eosin in order to perform histologic and histomorphometric studies under a stereoscopic microscope.
Digital microphotographs of the histologic sections of the hemi-mandibles were analyzed using the Image ProR Plus software, version 5.1 (Media Cybernetics) to measure the histomorphometric parameters listed below, based on stereologic principles 25 and using current nomenclature as stated by Parfitt 26 and revised by Dempster et al 27. Parameters measured in the alveolar bone of the developing alveolus (Fig. 1): * The degree of tooth eruption, expressed in millimeters, was determined as the distance between the highest point of the bone crest on the buccal aspect of the developing alveolus and the cementum-enamel junction. Therefore, the result is 0 when tooth eruption is complete and is a negative number when the tooth is not fully erupted. The following parameters were measured on both the buccal and lingual aspects. To simplify reading, only measures on the buccal aspect are explained in Fig. 1.
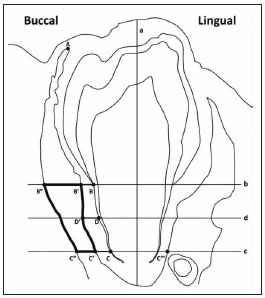
Fig. 1: Method used for the histomorphometric study of the developing tooth alveolus.
Buccal-lingual section.
Line a drawn through the longitudinal axis of the developing tooth.
Line b drawn perpendicular to line “a” through the cementumenamel junction.
Line c drawn perpendicular to line “a” through the diaphragm of Hertwig’s epithelial root sheath.
Line d drawn parallel to and equidistant from lines “b” and “c”.
Point A is the highest point of the bone crest.
Point B at the cementum-enamel junction.
Point C at the diaphragm of Hertwig’s epithelial root sheath.
Point D on line “d”, equidistant from points B and C on the surface of the developing root.
Point B´ drawn where line “b” goes through the periodontal plate of the developing alveolus.
Point C´ drawn where line “c” goes through the periodontal cortical plate of the developing alveolus.
Point D´, where line “d” goes through the periodontal cortical plate of the developing alveolus.
Point B´´, on line “b”, 100 microns from point B´.
Point C´´, on line “c”, 100 microns from point C´.
- The degree of tooth eruption was measured from point A to point B´.
- Periodontal width was determined, measuring segments BB ´ C- C´ D-D´.
- Bone volume was measured in the area delimited by points B´ C´ B´´ and C´´.
- Bone activity was assessed in three different regions of the alveolar wall:
- From point A to point C´
- From point C´ on the buccal aspect to point C´ on the lingual aspect
- On the endosteal walls in the area delimited by points B´ C´ E and F
* Periodontal width was measured at three sites: BB ´, C-C´and D-D´; values were averaged and results are expressed in microns.
* Bone volume, defined as the fraction of total volume corresponding to trabecular bone, was measured in the area demarcated by the black lines. Total volume is defined as the volume of trabecular bone tissue plus the volume of developing bone marrow; results are expressed as a percentage.
* Bone activity was evaluated in three different regions: on the wall of the developing alveolus from the highest point of the bone crest to the diaphragm of the Hertwig’s root sheath; on the wall of the fundus of the developing alveolus, from the diaphragm of Hertwig’s epithelial root sheath on the buccal aspect (Point C) to the diaphragm of Hertwig’s epithelial root sheath on the lingual aspect (Point C´´´); and on the endosteal walls, inside the area demarcated by the black lines, by determining the following parameters:
• ObS/BS (%): Bone formation surfaces (surfaces covered with active osteoblasts).
• ES/BS (%): Bone resorption surfaces (surfaces covered with active osteoclasts).
• LCS/BS (%): Resting bone surfaces, covered with bone lining cells.
Statistical analysis: The data were statistically analyzed using Student’s t test; statistical significance was set at a value of p <0.05.
Ethical principles: All procedures were performed in keeping with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (NIH publication 85-123 Rev.2010) and the study was approved by the Ethics Committee of the School of Dentistry of the University of Buenos Aires (FOUBA-UBACYT 2011-2014-3).
RESULTS
Our study showed that exposure to potassium dichromate in the form of chromium VI caused a significant delay in the eruption of the first lower molar, which was associated with a clear alteration in the development of the alveolus. The bone of the developing alveolus of experimental animals exhibited thinner and more spaced trabeculae than that of controls. Chromium-exposed animals showed fewer osteoclasts and active osteoblasts and more bone lining cells on the endosteal surfaces, the wall of the developing bone crest, and on the fundus of the alveolus (Fig. 2). The histomorphometric study showed significantly decreased bone volume in the alveolus of experimental animals compared to controls, on both the buccal and lingual aspects (Fig. 3).
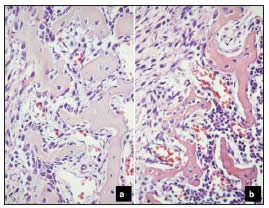
Fig. 2: Bone activity in the endosteal walls of the developing alveolus. Orig. Mag. X400. a: Control; b: Experimental. Note the large areas of bone formation with a large number of osteoblasts in the control section. Quiescent bone surfaces with inactive osteoblasts predominate in the experimental section; the latter also shows fewer and thinner trabeculae than the control section.
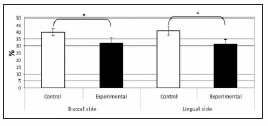
Fig. 3: Histomorphometric values of alveolar bone volume. Bone volume on both the buccal and lingual aspects was significantly lower in experimental animals than in controls (*p<0.05).
The surfaces of the endosteal bone trabeculae on the buccal and lingual aspects and fundus of the alveolus of the experimental group exhibited a lower percentage of bone formation and resorption surfaces and a higher percentage of resting surfaces than in the corresponding controls (Fig. 4).
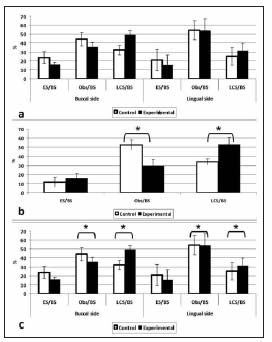
Fig. 4: Histomorphometric study of bone activity.
Bone activity in the wall of the developing alveolus.
Bone activity in the fundus of the alveolus.
Bone activity inside the developing alveolus.
A lower percentage of bone formation and resorption surfaces and a higher percentage of resting surfaces were observed in the buccal and lingual plates of the developing alveolar bone (c) and in the fundus of the alveolus (b) of experimental animals, as compared to controls. The differences between groups were statistically significant (* p<0.05). No significant differences in bone activity in the developing alveolus (a) were observed between groups.
The distance between the highest point of the bone crest on the buccal aspect of the developing alveolus and the cementum-enamel junction was greater in experimental animals than in controls, showing a significant delay in the eruption of the first lower molar. Hence, the result of the histomorphometric analysis was a negative number (Fig. 5). In addition, the periodontal space was narrower in the potassium dichromate-exposed animals than in controls, and the histomorphometric study showed it was significantly lower in experimental animals compared to controls, on both the buccal and lingual aspects (Fig. 6).
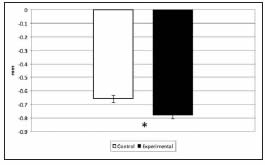
Fig. 5: Morphometric values of tooth eruption. The degree of tooth eruption was significantly lower in experimental animals compared to controls (* p<0.05).
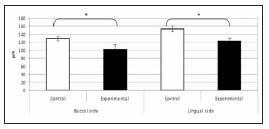
Fig. 6: Histomorphometric values of periodontal width. Periodontal width on both the buccal and lingual aspects was significantly lower in experimental animals as compared to controls (* p<0.05).
DISCUSION
The results of the present study showed that hexavalent chromium has a toxic effect on the bone cells involved in the bone remodeling process that takes place in the bone tissue of the developing tooth alveolus. According to De Lucca et al 17 this effect can be observed morphometrically as a delay in tooth eruption in animals exposed to potassium dichromate. It is well documented that bone remodeling involves the coupled action of osteoblasts (bone matrixforming cells) and osteoclasts (cells that resorb the bone matrix).
In vitro studies have reported that exposure to hexavalent chromium can affect human osteoblast and osteoclast survival and function 28. Thompson and Puleo 29 reported that chromium interferes in the differentiation and function of osteoblasts derived from mes enchymal cells. Ning and Grant 30 showed that hexavalent chromium reduced to trivalent chromium is a potent inducer of cytotoxicity in osteoblasts. Lohman et al 31 found changes in cell morphology and in the differentiation capacity of osteoblasts. In addition, a study by Anisian et al 32 showed that high concentrations of chromium decreased osteoblast activity. Nichols and Puleo 33 found that sub-lethal and physiological concentrations of hexavalent chromium affected the formation and function of osteoclasts. Thus, chromium would interfere in the differentiation of osteoclastic cells derived from precursor cells in the bone marrow, and would inhibit the Ca2+ receptors in osteoclasts. The receptor binding site is highly sensitive to diand tri-valent cations. Hence, given that hexavalent chromium reduces to trivalent chromium, the latter would bind to the receptor, increasing the cytosolic concentration of calcium and decreasing bone resorption. Neale et al 34 demonstrated the inhibitory effect of chromium on osteoclastogenesis in human monocytes cultured with chromium particles. Previous studies conducted at our laboratory showed that bone resorption and formation are indispensable during the intraosseous and mucosal penetration stages of tooth eruption, which is when the walls of the alveolus develop 21,22; the present results confirm those findings. The results of the our study show that exposure to hexavalent chromium leads to significantly decreased bone resorption and formation in the endosteal surfaces of the developing tooth alveolus and in the fundus of the alveolus, as shown by the presence of fewer osteoclasts and active osteoblasts. The larger proportion of areas of resting bone on the endosteal surfaces and fundus of the developing alveolus in experimental animals is similar to what occurs in adynamic bone disease. The latter disease has been observed for example, in cases of aluminum toxicity in humans 35 and in an experimental model of iron overload 18.
The present study showed decreased bone formation and resorption, and a predominance of bone quiescence in the developing alveolus of animals exposed to potassium dichromate as compared to controls. These findings show that hexavalent chromium affects bone turnover, as shown by the lower proportion of both areas of bone resorption andbone formation and the predominance of resting bone, which in turn results in the decrease of bone remodeling. These observations explain the delay in tooth eruption. Hexavalent chromium has been found to induce damage to the cytoskeleton36 and DNA alterations37 in exposed fibroblast cultures. These findings could explain the decreased periodontal width observed in the present study, which would be related to the inhibition of formation of fibroblasts that impairs periodontal ligament remodeling.
The results obtained in the present study allow concluding that because hexavalent chromium inhibits osteoclasts and osteoblast function, the delay in tooth eruption observed in animals exposed to hexavalent chromium would be due to a lower rate of bone remodeling in the developing tooth alveolus. Taking into account that in addition to the well known health consequences of hexavalent chromium exposure the latter can also affect tooth eruption in children who intake water contaminated with this toxic substance, it is crucial to create further awareness worldwide about the importance of avoiding environmental contamination, and ensuring compliance with waste-water treatment regulations with the aims to protect future generations.
ACKNOWLEDGMENTS
The authors thank Ht. Ivana Sanchez Rojas for her technical assistance and Ignacio Emanuel Sanchez for his collaboration in preparing the graphs of the manuscript. This study was supported by GRANT 2011-2104 UBACYT 20020100100196 (University of Buenos Aires).
1. Zhitkovich A. Chromium in drinking water: Sources, metabolism and cancer risks. Chem Res Toxicol 2011; 24:1617-1629. [ Links ]
2. Nath K, Kumar N. Hexavalent chromium: toxicity and its impact on certain aspects of carbohydrate metabolism of the freshwater teleost, Colisa fasciatus. Sci Total Environ 1988; 72:175-181. [ Links ]
3. Bocio A, Nadal M, Domingo JL. Human exposure to metals through the diet in Tarragona, Spain: temporal trend. Biol Trace Elem Res 2005; 104:193-201. [ Links ]
4. O´Brien TJ Ceryak S. Patierno SR. Complexities of chromium carcinogenesis: role of cellular response, repair and recovery mechanisms. Mutat 2003; 533: 3-36. [ Links ]
5. U.S. EPA. Toxicological review of hexavalent chromium. US Environmental Protection Agency 18549- 29-9, 1998. Washington DC. 70 pp. [ Links ]
6. Arauzo M, Rivera M, Valladolid M, Norea C, Cedenilla O. Contaminacion por cromo en el agua intersticial, en el agua del cauce y en los sedimentos del rio Jarama. Limnetica 2003; 22: 87-100. [ Links ]
7. Colombo JC, Barreda A, Bilos C, Cappelletti N, Demichelis S, Lombardi P, Migoya MC, Skorupka C, Suarez G. Oil spill in the Rio de la Plata estuary, Argentina: 1. Biogeochemical assessment of waters, sediments, soils and biota. Environ Pollut 2005; 134:277-289. [ Links ]
8. Banu SK, Samuel JB, Arosh JA, Burghardt RC, Aruldhas MM. Lactational exposure to hexavalent chromium delays puberty by impairing ovarian development, steroidogenesis and pituitary hormone synthesis in developing Wistar rats. Toxicol Appl Pharmacol 2008; 232:180-189. [ Links ]
9. Cuberos E., Rodriguez AI, Prieto E. Chromium levels and their relationship with alterations in the health of tannery workers living and working in Bogota, Colombia. Rev Salud Publica (Bogota) 2009; 11:278-289. [ Links ]
10. Tziritis E, Kelepertzis E, Korres, G, Perivolaris D, Repani S. Hexavalent chromium contamination in croundwaters of Thiva Basin, central Greece. Bull Environ Contam Toxicol 2012; 89:1073-1077. [ Links ]
11. Greenpeace. Informe: Cueros toxicos. Nuevas evidencias de contaminacion de curtiembres en la cuenca Matanza- Riachuelo 2012; 27 pp. [ Links ]
12. Costa M. Toxicity and carcinogenicity of Cr (VI) in animal models and humans. Crit Rev Toxicol 1997; 27: 431-442. [ Links ]
13. Sutherland JE, Zhitkovich A, Kluz T, Costa M. Rats retain chromium in tissues following chronic ingestion of drinking water containing hexavalent chromium. Biol Trace Elem Res 2000; 74:41-53. [ Links ]
14. Sankaramanivel S, Jeyapriya R, Hemalatha D, Djody S, Arunakaran J, Srinivasan N. Effect of chromium on vertebrae, femur and calvaria of adult male rats. Hum Exp Toxicol 2006; 25:311-318. [ Links ]
15. Agency for Toxic Substances and Disease Registry. Toxicological Profile for Chromium. U.S. Department of Health and Human Services, Washington 2012. D.C. 497 pp. [ Links ]
16. Soudani N, Ben Amara I, Troudi A, Bouaziz H, Boudawara T, Zeghal N. Oxidative stress induced by chromium (VI) in bone of suckling rats. Toxicol Ind Health 2011; 27:724-734. [ Links ]
17. De Lucca RC, Dutrey PL, Villarino ME, Ubios AM. Effect of different doses of hexavalent chromium on mandibular growth and tooth eruption in juvenile Wistar rats. Exp Toxicol Pathol 2009; 61:347-352. [ Links ]
18. Mandalunis P, Ubios A. Experimental renal failure and iron overload: a histomorphometric study in rat tibia. Toxicol Pathol 2005; 33:398-403. [ Links ]
19. Conti MI, Terrizzi AR, Lee CM, Mandalunis PM, Bozzini C, Pineiro AE, Martinez MP. Effects of lead exposure on growth and bone biology in growing rats exposed to simulated high altitude. Bull Environ Contam Toxicol 2012; 88: 1033-1037. [ Links ]
20. Ubios AM, Braun EM, Cabrini RL. Effect of diphosphonates on abnormal mandibular growth of rats intoxicated with uranium. Health Phys 1998; 75:610-613. [ Links ]
21. Pujadas Bigi MM, Lemlich L, Mandalunis PM, Ubios AM. Exposure to oral uranyl nitrate delays tooth eruption and development. Health Phys 2003; 84:163-169. [ Links ]
22. Villarino ME Goya JA, De Lucca RC, Ubios AM. Alterations of tooth eruption and growth in pups suckling from diabetic dams. Pediatr Res 2005; 58:695-699. [ Links ]
23. Wise GE, He H, Gutierrez DL, Ring S, Yao, S. Requirement of alveolar bone formation for eruption of rat molars. Eur J Oral Sci 2011; 119:333-338. [ Links ]
24. Tiwari R, Saha A, Sathwara N, Parikh J. Blood chromium levels of children working in gem-polishing industries in India. Toxicol Ind Health 2012; 28:170-173. [ Links ]
25. Weibel E, Elias H. Quantitative methods in morphology. In: Weibel ER, Elias H (eds). Introduction to stereology and morphology. Springer Verlag, Berlin 1967; 3-19. [ Links ]
26. Parfitt AM. Bone histomorphometry: standardization of nomenclature, symbols and units (summary of proposed system). Bone 1988; 9:67-69. [ Links ]
27. Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2013; 28:2-17. [ Links ]
28. Andrews R, Shah K, Wilkinson J, Gartland A. Effects of cobalt and chromium ions at clinically equivalent concentrations after metal-on-metal hip replacement on human osteoblasts and osteoclasts: Implications for skeletal health. Bone 2011; 49:717-723. [ Links ]
29. Thompson GJ, Puleo DA. Effects of sub-lethal metal ion concentrations on osteogenic cells derived from bone marrow stromal cells. J Appl Biomater 1995; 6:249-258. [ Links ]
30. Ning J, Grant MH. Chromium (VI)-induced cytotoxicity to osteoblast-derived cells. Toxicol in Vitro 1999; 13: 879-887. [ Links ]
31. Lohmann CH, Schwartz Z, Koster G, Jahn U, Buchhorn GH, MacDougall MJ, Casasola D, Liu Y, Sylvia VL, Dean DD, Boyan BD. Phagocytosis of wear debris by osteoblasts affects differentiation and local factor production in a manner dependent on particle composition. Biomaterials 2000; 21:551-561 [ Links ]
32. Anissian L, Stark A, Dahistrand H, Granberg B, Good V, Bucht E. Cobalt ions influence proliferation and function of human osteoblast-like cells. Acta Orthop Scand 2002; 73:369-374. [ Links ]
33. Nichols KG,Puleo DA. Effect of metal ions on the formation and function of osteoclastic cells in vitro. J Biomed Mater Res 1997; 35:265-271. [ Links ]
34. Neale SD, Haynes DR, Howie DW, Murray DW, Athanasou NA. The effect of particle phagocytosis and metallic wear particles on osteoclast formation and bone resorption in vitro. J Arthroplasty 2000; 15:654-662. [ Links ]
35. Malluche HH. Aluminium and bone disease in chronic renal failure. Nephrol Dial Transplant 2002; 17:21-24. [ Links ]
36. Rudolf E, Cervinka M. Nickel modifies the cytotoxicity of hexavalent chromium in human dermal fibroblasts. Toxicol Lett 2010; 197:143-150. [ Links ]
37. Liu W, Chaspoul F, Botta C, De Meo M, Gallice, P. Bioenergetics and DNA alteration of normal human fibroblasts by hexavalent chromium. Environ Toxicol Pharmacol 2010; 29:58-63. [ Links ]














