INTRODUCTION
Contrast-induced nephropathy (CIN) may develop after coronary angiography due to intravascular administration of iodinated contrast agents in susceptible individuals. In the United States, approximately one million coronary angiographies are performed annually, and this number is about 335,000 per year in our country (1,2). The incidence of CIN has been reported to be lower than 3% in patients with normal renal function; however, this rate rises to 12-40% in the presence of additional risk factors (3,4). CIN is the third most common cause of hospital-acquired acute kidney injury (AKI) (5).
Although there is a high level of quality evidence related to intravenous hydration, the use of non-ionic, low-osmolar, or iso-osmolar contrast agents, there are also applications with lower levels of evidence such as the use of oral n-acetylcysteine and high-dose statin in preventing CIN (6,8).
Remote ischemic preconditioning (RIPC) is a non-invasive, easy-to-apply, and inexpensive method with few side effects used to protect a remote organ or tissue from a prolonged episode of ischemia/reperfusion injury via applying one or more brief episodes of ischemia to an organ or tissue. Since 1986, RIPC application has been shown to reduce organ damage in several studies (9,14). RIPC is thought to protect by activating various anti-inflammatory, antioxidant, neuronal, and humoral pathways (15). However, its mechanism has not been clarified yet.
Although RIPC can be an effective tool to protect kidneys from ischemic injury based on experimental and clinical evidence, the ideal areas of application (arm, leg, internal organ) have not been clarified yet. Wever KE. et al. demonstrated that bilateral RIPC was more effective than unilateral RIPC in an animal experiment, but clinical trials in humans have not been conducted (16). Therefore, we compared alterations in renal functions and the rate of development of CIN after coronary angiography by applying RIPC in the lower and upper limbs of patients undergoing coronary angiography due to stable angina pectoris.
METHODS
Study Population
We started this single center, randomized controlled, and prospective pilot study after obtaining approval from the University of Health Sciences Haseki Training and Research Hospital Ethics Committee (approval number:406/19.10.2016).>
We included the patients who underwent coronary angiography because of stable angina pectoris, who were over 18 years of age, and provided written informed consent. The exclusion criteria were as follows: acute myocardial infarction within the last 7 days, clinical signs of unstable angina pectoris, patients who were administered intravenous or intra-arterial contrast agent within the last 4 weeks, AKI in the last three months, pregnant women, patients having peripheral vascular disease affecting the upper or lower extremity, any advanced systemic disease, a recent history of nephrotoxic drug use or diuretic use, patients who were administered contrast agent below 30 ml were excluded.
A total of 168 adult patients who gave informed consent and underwent coronary angiography due to stable angina pectoris in the Cardiology Clinic at Haseki Training and Research Hospital were enrolled in the study. We randomized patients to RIPC groups (arm, leg, and control group) according to the order in which they came to the coronary angiography unit. 60 patients were randomized to the upper limb group, 58 patients to the lower limb group, and 50 patients to the control group.
Application of Research and Data Collection:
All demographic features of patients were recorded. We asked the patients not to consume tea/coffee and smoke at least 30 minutes before the measurement, blood samples were taken, and we placed them in a quiet environment to rest for half an hour and measure blood pressure. We divided the patients into 4 categories according to the Mehran risk score used for the prediction of CIN after percutaneous coronary intervention. Mehran risk score is a scoring system based on 8 variables: age >75 years, hypotension, intra-aortic balloon pump, congestive heart failure, chronic kidney disease, diabetes, anemia, and volume of contrast. A score of 0-5 was scored as a low risk, a score of 6-10 was grouped as moderate risk, a score of 11-15 was grouped as high risk and, a score of 16 or above was grouped as a very high-risk group 17. Patients who had a high risk for developing CIN according to the Mehran score were given 1 mL/kg/h intravenous isotonic saline for a total of 12 hours, 6 hours before and 6 hours after the procedure.
RIPC Application: We randomized patients to RIPC groups (arm, leg, and control group) according to the order in which they came to the coronary angiography unit. The blood pressure was measured on both arms and legs with the correct size cuff according to the manufacturer’s instructions. The thigh was used for the lower extremity and the arm was used for the upper extremity. The dominant limb was preferred for the extremity to be used. RIPC consisted of 3 cycles of 5-minute inflation of a blood pressure cuff to 50 mmHg above the resting systolic arterial blood pressure to one lower extremity or upper extremity followed by 5-minute reperfusion with the cuff deflated 18. In the control group, the cuff was applied to the patient's dominant arm but the inflation process was not performed.
Coronary Angiography: The physicians, who were blinded to the study groups, performed all coronary angiography procedures according to the standard using Iohexol (KOPAQ) non-ionic low-osmolar contrast agent. The volume of contrast agent used during the procedure was recorded in mL, by obtaining information from the physician performing coronary angiography. The time between the end of the RIPC procedure and the beginning of the coronary angiography was between 10 and 45 minutes.
Follow-up and Definitions: Within 48-72th hours after the procedure, we measured serum creatinine levels of all patients, inpatients at the hospital while other patients were called from home. Δ percent in creatinine levels of patients were calculated with the formula (48-72th hours creatinine / initial creatinine x 100).
According to the European Society of Urogenital Radiology (ESUR) guidelines on contrast agents, an increase in serum creatinine by more than 25% or 0.5 mg/dL indicates CIN. Acute Kidney Injury Network (AKIN) criteria defined contrast-induced AKI as an increase in serum creatinine by more than 0.3 mg/dL and/or 50% from baseline (19,20). The estimated glomerular filtration rate (eGFR) was calculated by the modification of diet in renal disease (MDRD) using the formula (21). The primary outcome of our study was to compare the rate of prevention of CIN between lower limb and upper limb RIPC. Secondary outcomes were as follows: To evaluate the rate of CIN and prevention of CIN using RIPC compared to the control group, the effect of RIPC on Δ creatinine levels, duration of hospitalization, myocardial infarction status during the procedure and on mortality during hospitalization as well side effects associated with RIPC.
STATISTICAL ANALYSIS
The analyses were performed by using the SPSS software (Statistical Package for the Social Sciences, version 20.0, SPSS Inc, Chicago Illinois, USA). Descriptive statistics; numbers and percentages were given for categorical variables and mean and standard deviation for numerical variables. The Chi-square test was used for the comparison of the ratios between the groups. The independent sample t-test was used for the comparisons between the two independent groups when the numerical variables provided the normal distribution and Student’s t-test was used for the dependent groups if the normal distribution condition was provided. Comparisons between three independent groups were performed by ANOVA test when the numerical variables provided the normal distribution condition. The independent predictors of CIN were evaluated using multivariate Binary regression analyses. The Binary logistic models included demographic and clinical parameters that suggested a potential effect on the development of CIN in univariate analyses. The statistically significant independent predictors of CIN were determined by the Binary regression using the “enter method”. A p-value of less than 0.05 was considered statistically significant
RESULTS
Baseline Data
The study was completed with 168 adult patients (60 patients to upper limb RIPC, 58 patients to lower limb RIPC, and 50 patients to control group) with a mean age of 59.6 ± 9.1 years; 15 of 183 patients were excluded from the study because it could not be obtained a control blood sample; 95 (56.5%) patients were male. The median Mehran risk score was 4 (IQR 1-5) and the mean was 3.5±2.5. According to the Mehran risk score, 135 (80.4%) patients belonged to the low-risk, 31 (18.5%) to moderate-risk, and 2 (1.2%) to the high-risk category. No patient was having a very high risk for CIN. The median duration between RIPC and coronary angiography was 20 (IQR 15-30) minutes and the median dose of intravenously administered contrast medium was 80 (IQR 50-100) mL.
Remote Ischemic Preconditioning versus Control
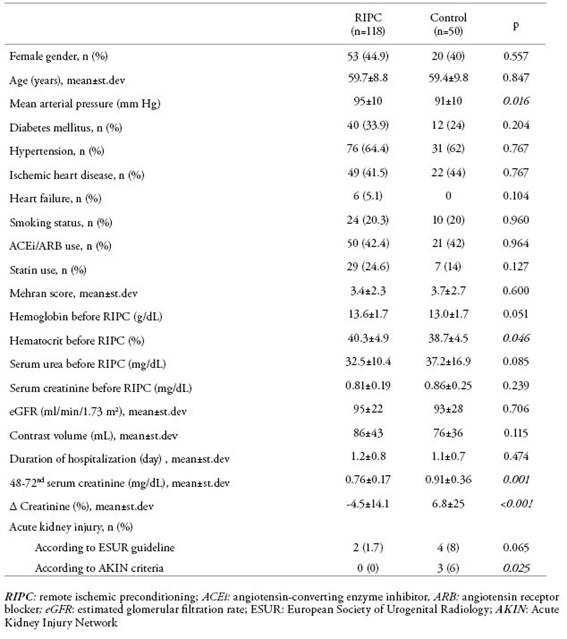
RIPC was performed in 118 of 168 patients and 50 patients served as controls. Data of the patients before and after the procedure are given inTable 1. Using the ESUR criteria, AKI incidence was found in 1.7% of 118 patients who underwent RIPC, while 8% of patients in the control group (OR: 5.053; 95% CI: 0.893-28.488; p=0.065). According to the AKIN criteria, none of the 118 patients who underwent RIPC had CIN whereas, in the control group, AKI developed in 6% of patients. (OR: 3.511; 95% CI: 2.757-4.471; p=0.025). Also, serum creatinine levels at the 48-72th hours after coronary angiography were significantly higher in controls than in RIPC patients (p=0.001). Creatinine levels were increased by 32% and 54% of RIPC patients and controls, respectively (p=0.008). No complication was observed during RIPC except pain and tingling in the extremity.
Table 1: Comparison of data of the patients and the control group before and after RIPC
RIPC: remote ischemic preconditioning; ACEi: angiotensin-converting enzyme inhibitor, ARB: angiotensin receptor blocker; eGFR: estimated glomerular filtration rate; ESUR: European Society of Urogenital Radiology; AKIN: Acute Kidney Injury Network
Upper Limb versus Lower Limb
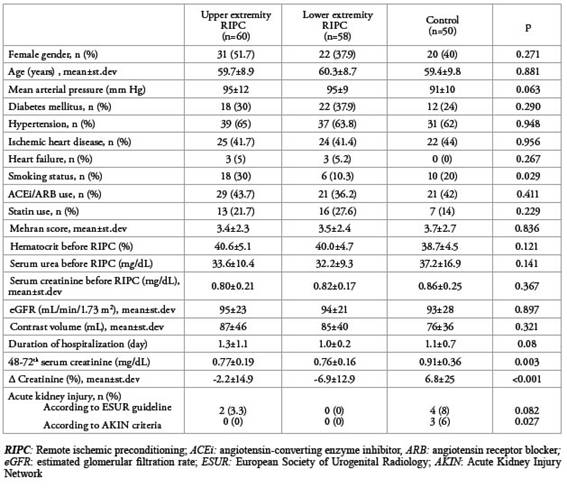
The mean inflation pressure in patients who underwent upper limb RIPC was 182±16 mmHg and the mean inflation pressure in patients who underwent lower limb RIPC was 203±12 mmHg.shows the comparison of the general characteristics of patients who underwent upper limb RIPC, lower limb RIPC and patients without RIPC as the control group.Table 2
Table 2: Comparison of data of the RIPC applied to patients on upper and lower extremities and the control group.
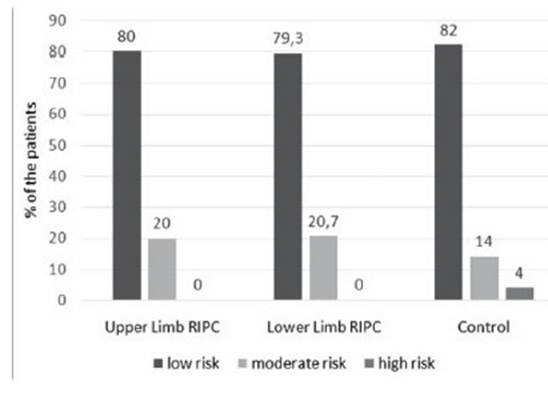
Mehran scores and eGFR before coronary angiography were found to be similar between lower and upper-limb RIPC patients and controls. While 48 (80%) of upper limb RIPC patients had a lower risk of developing CIN according to the Mehran score, this rate was 79.3% and 82% in lower limb RIPC patients and controls, respectively (p = 0.937) (Figure 1).
Figure 1: Category of Mehran scores before coronary angiography in lower and upper limb RIPC patients and controls. According to the Mehran score, there was no significant difference between the groups employing the development of CIN before coronary angiography (p= 0.937).
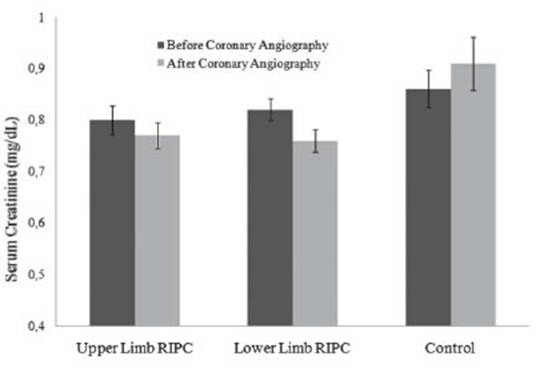
When the three groups were compared with each other, there was no significant difference in the duration of hospitalization. The 48-72th hour serum creatinine level in the control group was significantly higher than in the other two groups, however, there was no significant difference between lower limb and upper limb RIPC patients (p=0.003 and p=0.935, respectively). According to the ESUR guideline, there was no significant difference in the development of CIN between the three groups (p=0.082). However, when we compared according to the AKIN criteria, the incidence of CIN was significantly higher in the control group than in the other two groups; the rates were similar between lower limb and upper limb RIPC patients (p=0.027 and p<0.99, respectively). When the creatinine levels in the three groups were compared with the baseline values, the mean creatinine level was found to be increased in the control group and decreased in the other two groups (p<0.001) (Figure 2). Also, the percentage of creatinine change after lower limb and upper limb RIPC was similar (p=0.322).
Figure 2: The creatinine levels before and after coronary angiography in lower and upper limb RIPC patients and controls. The mean creatinine level was found to be increased in the control group and decreased in the other two groups compared with the baseline values (p<0.001)
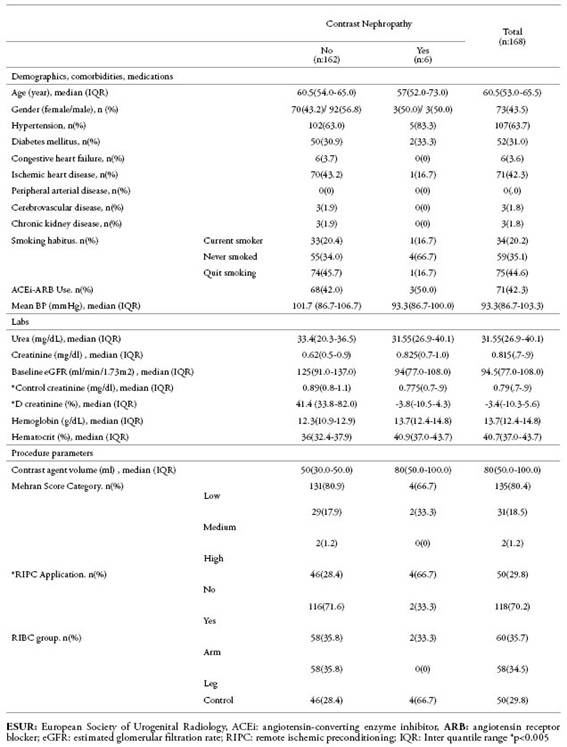
InTable 3, we also presented the characteristics and laboratory data of the patients with the development of CIN according to the ESUR criteria. The only significant difference between patients who developed CIN and those who did not was the application of RIPC.
Table 3: The characteristics and lab values of the patients according to the development of contrast-induced nephropathy (CIN) defined by ESUR. We considered CIN as an increase in baseline serum creatinine greater than 0.5mg / dL or greater than 25% in control samples taken 48-72 hours after coronary angiography.
ESUR: European Society of Urogenital Radiology, ACEi: angiotensin-converting enzyme inhibitor, ARB: angiotensin receptor blocker; eGFR: estimated glomerular filtration rate; RIPC: remote ischemic preconditioning; IQR: Inter quantile range
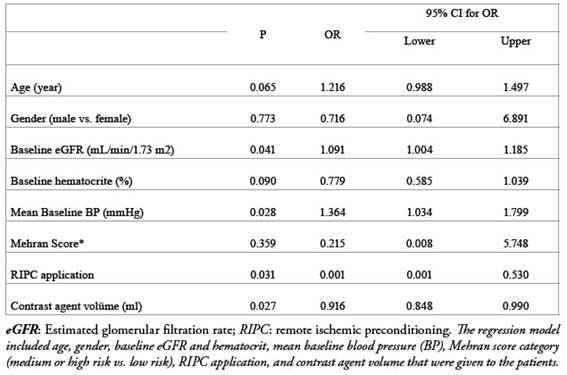
We performed a multivariate analysis to reveal independent factors related to the development of CIN using a regression model including age, gender, baseline eGFR and hematocrit, mean baseline blood pressure (BP), Mehran score category (medium or high risk vs. low risk), RIPC, and contrast agent volume and found out that baseline eGFR and mean blood pressure, contrast agent volume and, RIPC application were independently associated with the development of CIN (Table 4).The regression model included age, gender, baseline eGFR and hematocrit, mean baseline blood pressure (BP), Mehran score category (medium or high risk vs. low risk), RIPC application, and contrast agent volume that were given to the patients.
Table 4: Multivariate analysis of the factors for contrast-induced nephropathy.
DISCUSSION
The kidney which is particularly susceptible to ischemic injury with its high energy requirements and the complex microvascular network is one of the major organs used in RIPC’s clinical practice. Although experimental and clinical evidence suggest that RIPC can be an effective way to protect kidneys from ischemic injury, the fact that the ideal areas of application of the method (arm, leg, internal organs) have not been clarified is an important deficiency in this issue. In our study, we demonstrated that RIPC may be an effective method in preventing CIN after coronary angiography. But we did not find any difference between the RIPC application sites (arm vs. leg). The fact that there has been no study comparing the application site for this method makes our study valuable.
In our study, the incidence of AKI was found to be 3.6% and the most important reason for this low rate may be the fact that 80% of patients in our patient group were in the low-risk group according to the Mehran score. In our study, only two patients were in the high-risk group and two patients had premedication (i.v. hydration) and AKI did not develop in these patients. AKI was seen in 1.7% of RIPC patients and 8% of controls. In addition, creatinine levels after the procedure were significantly lower in RIPC patients than in controls. The percentage of creatinine change after the procedure was also higher in the control group. In multivariate analysis, RIPC was found to be an independent factor affecting the development of CIN. All of these results suggest that preoperative RIPC may be an effective method for the prevention of CIN developing after coronary angiography in low- and medium-risk groups of patients. First, in 1986, Murry et al. described the cardioprotective effect of short ischemic attacks after prolonged ischemia in dogs and revealed that it can be used effectively in different organs 9. In the RenPro-Trial study, Er et al. showed that RIPC dramatically decreased the risk of CIN after invasive coronary intervention in patients with chronic kidney disease who had a high Mehran score (12% in the RIPC group, 40% in the control group, p=0.002) 10. In a study conducted by Deftereos et al., it was observed that RIPC decreased CIN after coronary angiography in patients with a median Mehran score of 10 (12.4% in the RIPC group, 29.5% in the control group; p=0.002) (11). Yamanaka et al. also found a low rate of CIN after coronary angiography in patients with a mean Mehran risk score of 7.8 ± 6.0 and 7.4 ± 5.7 and reported that the incidence of CIN was 10% in the RIPC group and 36% in the control group (p=0.003) (12). Recently, two randomized controlled studies and meta-analysis reported that RIPC after percutaneous coronary intervention significantly reduced the incidence of AKI (12,14,22). Although there have been randomized controlled trials showing RIPC to be an effective method of preventing CIN after coronary angiography in risky patient groups, there are also some studies that did not have favorable results about RIPC application to prevent CIN. In their study, Arash Gholoobi et al. reported that there was no significant difference in the incidence of CIN between RIPC patients and controls and that adequate fluid therapy was still the most effective method of prevention of CIN (23). There are also meta-analysis showing the same conclusion (24). Menting et al. reported that RIPC application was ineffective in preventing CIN but in subgroup analysis, it was found to be effective, especially in patients whose Mehran risk score was 11 and above (25). In a meta-analysis in which the results of nine recent studies were evaluated, it was reported that RIPC may be effective in preventing CIN (6.5% in RIPC, 13.5% in the control group (RR 0.430, 95% CI 0.286-0.648; p=0.000) (26). In our study, the incidence of CIN was found to be lower in RIPC patients than in controls (according to the ESUR criteria, 1.7% in RIPC and 8% in controls, OR: 5.053; 95% CI: 0.893-28.488; p=0.065 and, according to AKIN criteria, 0% in RIPC and 6% in controls OR: 3.511; 95% CI: 2.757-4.471; p=0.025). While pre-operative creatinine levels were similar between both groups, post-operative creatinine level was significantly higher in the control group (0.76±0.17 mg/dL in RIPC groups and 0.91±0.36 mg/dL in controls; p=0.001). In addition, creatinine levels in RIPC patients decreased by -4.5±14.1% compared to baseline, whereas creatinine levels increased by 6.8±25% compared to baseline in the control group (p<0.001). All of these results suggest that RIPC may be an effective method to prevent CIN after coronary angiography in patients in low- or moderate-risk groups according to the Mehran score.
The most important point that confuses the mind about the clinical application of the RIPC method in routine practice is the lack of a standardized optimal protocol for the delivery of RIPC. Different results between studies cause problems in comparison. In a meta-analysis performed by Hu et al., in 22 of the 30 studies included in the study, RIPC was done in the upper limb, in the lower limb in 5, and both upper and lower limbs in 1, and the iliac artery was clamped in two studies (26). In our study, we compared the results of lower and upper limb RIPC and we found similar creatinine levels after lower limb and upper limb RIPC (p=0.935). The ratio of AKI development and the percentage of change in creatinine levels after RIPC administration to the arm and the leg was similar (p=0.322). These results have shown that lower limb and upper limb RIPC had similar efficacy in preventing CIN.
Since the benefit of RIPC has been reported to be more prominent in high-risk patients in previous studies, we assume that our study includes low- and medium-risk patients and revealing the effectiveness of the method in these patient groups may contribute to further studies. In this way, we aimed to show that this application may be widely used for preventing CIN in all patients undergoing coronary angiography. However, being non-invasive, cheap, and easy to apply differs RICP from other recommendations. The absence of any side effects rather than pain and tingling is also an important advantage.
The main limitation of our study was a single-center pilot study. Because the primary objective of the study was the comparison of the upper and lower extremities, to reduce the effect of other variables to standardize the method, our study was planned as a single center. Second, as this was a pilot study, the number of patients was limited and therefore these findings should be confirmed in a larger prospective study.
As a result, RIPC is a non-invasive method that can be applied easily without serious side effects. It is especially useful in preventing CIN in low or medium-risk groups. Applying RIPC on the leg or arm has a similar effect. Studies on the effectiveness of RIPC application in patient groups with high CIN risk are needed.