Introduction
Diarrhoeal diseases are a major cause of infant morbidity and mortality and constitute a public health problem in the paediatric population of developing countries [1-3].
In Argentina, acute diarrhoea accounts for 60-80% of paediatric consultations in health services[4]. About 1.2 million cases are reported in hospitals each year [5], with Shigella as the main aetiological agent, followed by Salmonella and diarrhoeagenic E. coli [6, 7, 8].
This disease is generally self-limiting so that only adequate hydration is needed. In severe situations, prompt antibiotic treatment can control symptoms and reduce the spread of infection [9]. The World Health Organisation (WHO) recommends antibiotic treatment in these cases, based on local resistance patterns [10].
The epidemiological situation of diarrhoea is not static. Aetiological agents may vary according to demographic, environmental, geographical, seasonal, climatic or behavioural factors, and epidemiological surveillance is therefore a useful tool for understanding the current situation [11, 12]. For this information to be used to generate impact measures, it is essential to integrate it with information from laboratory surveillance of circulating serotypes and antibiotic resistance [13]. Organisms of the genus Shigella belong to the family Enterobacteriaceae. They are Gram-negative bacilli 0.3 to 1 μm in diameter and 1 to 6 μm in length, facultative anaerobes, non-spore-forming, non-gas-producing and non-motile; they cause the disease known as shigellosis or bacillary dysentery [7, 8].
The aim of this study was to evaluate the presence and microbiological characteristics of acute diarrhoeal disease caused by Shigella in children treated at the "Dr. Fernando Barreyro" Paediatric Hospital in Posadas, Misiones, between January 2016 and December 2019.
This study is part of the accredited framework project "Pathogens associated with acute diarrhoeal disease (ADD) in children in Posadas Misiones. Epidemiology, etiology and microbiological characteristics" (16Q639) Faculty of Exact, Chemical and Natural Sciences of the National University of Misiones.
Materials and methods
A prospective descriptive cross-sectional study was conducted. We included 106 Shigella spp isolates from stool cultures of children seen at the Paediatric Hospital between January 2016 and December 2019.
Faecal samples (FM) were processed according to stool culture protocols [14] and isolates were identified by conventional biochemical tests [15].
Seroaggregation was performed with antisera provided by INEI-ANLIS "Dr. Carlos G. Malbrán".
Antimicrobial susceptibility was determined according to the WHONET surveillance protocol and based on Clinical and Laboratory Standards Institute guidelines [16]. The antibiotics tested from Laboratorios Britania, Argentina were ampicillin (AMP, 10 μg), ciprofloxacin (CIP 5 μg), cefpodoxin (CPD 10μg), nalidixic acid (NAL 30μg), trimethoprim-sulfamethoxazole (TMS, 1.25/23.75μg) and nitrofurantoin (NIT 300 μg) for furazolidone evaluation. Multidrug resistance was defined as resistance to 3 or more unrelated antibiotic families.
The following age groups were considered: infants: less than or equal to 2 years, preschool: between 3-5 years, school: older than 5 years.
Data were recorded in an Excel 2007 spreadsheet and analysed with the EPI INFO 7 software. An error rate of α of 5% was accepted, where a value of p<0.05 was considered statistically significant.
We worked with the approval of the Bioethics Committee and the Teaching and Research Department of the Paediatric Hospital.
Results
During the study period, 2,128 faecal samples were processed and 106 (5%) Shigella spp. isolates were recorded; S. flexneri was the most frequently detected species with 64 isolates (60.4%), followed by S. sonnei 37 (34.9%). No cases of S. dysenteriae or S. boydii were observed. Five isolates were found to be non-typeable. In 2016 we observed an increase in recovery of this pathogen, with a slight percentage decrease of S. flexneri, although without statistically significant differences (Table 1).
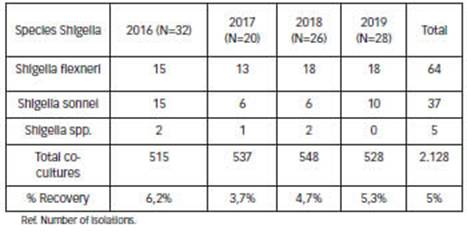
Table 1: Frequency of annual recovery of Shigella spp. Provincial paediatric hospital (period 2016- 2019). (N=106) Ref. of isolations.
S. flexneri 2 (54 isolates) was the main serotype detected. Three isolates of serotype 3 were also characterised, two as serotype AA479 and one isolate as serotype 1.
The highest number of cases of both species was detected in the warm months: summer (53%), autumn (23%) and spring (17%) (Figure 1).
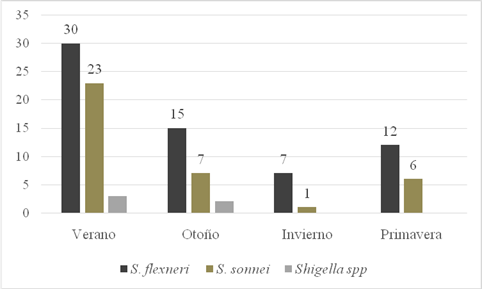
Fig. 1: Seasonal distribution of Shigella isolates. Provincial Paediatric Hospital "Dr. Fernando Barreyro". Period (2016-2019)
When we analysed the ages of the patients we found that the mean age was 4.2 years, with a median of 4 years (1.2 months-11 years). We recorded 41 infants, 31 preschoolers and 34 schoolchildren. Infants and preschoolers were the most affected children with almost 70 % of the total recorded.
Ninety-eight patients were outpatients and 8 (7.5%) required hospitalisation for treatment. There was a marked predominance of shigellosis in outpatients; between outpatients and those requiring hospitalisation for treatment, there was no statistically significant relationship with age group (p=0.5161). However, we found that S. flexneri predominated strongly in the infant group and S. sonnei in school children (Figure 2).
Overall resistance to AMP and TMS was high (greater than 50%). More than 97% of isolates were sensitive to FUR, CPD and quinolones: CIP and NAL (Table 2).
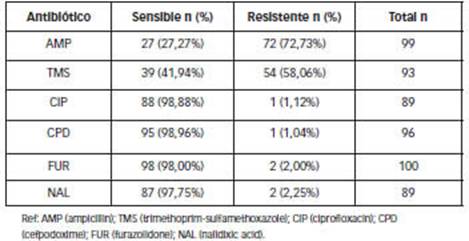
Table 2: Percentages of overall sensitivity and resistance of Shigella spp to antimicrobials. Patients attended at the Hospital Provincial de Pediatría "Dr. Fernando Barreyro". Period (2016-2019). (n=106)
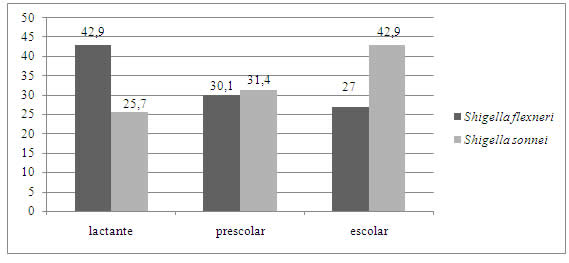
Fig.2: Percentagesof affected age groups according to species. Provincial Paediatric Hospital "Dr. Fernando Barreyro". Period (2016-2019). Ref: infants (1 month-2 years); preschoolers (3-5 years); schoolchildren (>5 years).
S. flexneri showed higher resistance to AMP (78.7%) than S. sonnei (61.8%), with no significant statistical difference (p=0.5063). S. sonnei showed higher resistance than S. flexneri to TMS (80.6% vs. 44.8%, respectively) with statistically significant difference (p=0.0053).
We detected 6 different phenotypes among the 77 isolates on which all drugs were tested; phenotype 1 was predominant (50.6%; table 3). Thirteen isolates (16.8%) were found to be sensitive to all antimicrobials tested (phenotype 3). Resistance to at least one antibiotic was observed in 29.8% of the isolates (phenotypes 2 and 4), 50.6% to two (phenotype 1) and only two isolates (2.6%) were multi-resistant (phenotype 5 and 6).
Discussion
Several authors have shown that the prevalence of Shigella species is variable between different regions or countries, depending on several factors, including climatic and socioeconomic aspects [12].
The percentage of confirmation of Shigella as an aetiological agent of acute diarrhoea, by conventional methods, is similar to that reported by other authors [17, 18].
When analysing our results, we observed that the same pattern of species shown by developing countries is maintained, as reported for other Latin American countries [19-21] and other Argentine provinces [11, 17, 22-25] in the years 2020, 2019, 2017, 2012 and 2007 respectively, where S. flexneri predominated with a percentage between 60 and 70%, followed by S. sonnei.
The increase in the prevalence of S. sonnei, detected in 2016, was observed in recent years in some Latin American countries, which have experienced socio-economic improvements [26]. Such an increase is emerging as a problem in developing areas experiencing public health and water quality improvements [27]. This phenomenon has been well documented in China [28], Iran [29] and Brazil [30] in areas that have experienced rapid industrialisation.
In general, the literature reports that most cases of Shigella occur in the warmer months [11, 31] in agreement with our findings.
The age range of affected patients and the calculated median are similar to those obtained in studies conducted in paediatric hospitals in Argentina [11, 24] and in developing countries [22, 25].
The detection of S. flexneri as the predominant species only among younger children differs from that reported by other researchers who indicate that S. flexneri is the most isolated species in all age groups [22, 24].
As this is a self-limiting disease, several authors highlight the low frequency of patients requiring hospitalisation for this cause [14, 23] in all age groups.
The high resistance detected for ampicillin and trimethoprim-sulfamethoxazole and almost nil for ciprofloxacin is similar to surveillance results reported by the WHONET- SIREVA II Network (2020) for the period 2010-2019 as well as with different studies from Argentina [11, 23], Ecuador [20] and Peru [21].
The profiles found are similar even in Asia. In a study conducted in India, clear resistance to ampicillin, trimethoprim/sulfamethoxazole and nalidixic acid was observed as a predictor of quinolones [32].
Higher resistance of S. flexneri to ampicillin compared to S. sonnei and vice versa for trimethoprim-sulfamethoxazole were also described by the WHONET/ SIREVA II Network (2020) for our country, during the period 2016 to 2019 [33]. However, it differs from the findings of Luque A. et al. (2015)[21], where ampicillin resistance levels were significantly higher in S. sonnei isolates compared to other Shigella serogroups.
The emergence of multidrug-resistant strains makes the choice of antibiotic treatment for shigellosis difficult and has become a public health problem in several places [34, 35].
Despite the different study designs, it is important to note the low proportion of multidrug-resistant isolates found in our study, especially considering the high frequency of these strains found by other authors, such as 50% [36] or 80% [37]. This low proportion is favourable in terms of behaviour and the possible therapeutic range, and means that the treatment of choice at the Paediatric Hospital follows the recommendations of the Argentine Paediatric Society, with the use of furazolidone as the first line of treatment in outpatients [7].
Conclusions
The presence of Shigella spp. was documented in 5% of stool cultures; children < 5 years were the most affected (70 %) and 7.7% required hospitalisation. The most frequent species was S. flexneri followed by S. sonneii. Antimicrobial resistance profiles were similar to those detected in Argentina, with high levels of resistance to ampicillin and Trimethoprim/sulfamethoxazole (above 15%) although multidrug resistance was rare (2%). Furazolidone remains the antimicrobial of choice for outpatient treatment of acute diarrhoeal disease caused by this species in children in the region. Surveillance of these agents of acute diarrhoeal disease should be continued to obtain accurate information for correct antibiotic use.















