Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Acta Odontológica Latinoamericana
versión On-line ISSN 1852-4834
Acta odontol. latinoam. vol.22 no.1 Buenos Aires abr. 2009
ARTÍCULOS ORIGINALES
Frequency and distribution of Mutans Streptococci in dental plaque from caries-free and caries-affected Venezuelan children
Ana M. Acevedo, Maria V. Ray, Mairobys Socorro, Fátima Rojas-Sánchez
Institute of Dental Research, School of Dentistry, Central University of Venezuela, Caracas, Venezuela.
CORRESPONDENCE Dr. Ana Maria Acevedo Instituto de Investigaciones Odontologicas “Raul Vincentelli” Facultad de Odontologia Universidad Central de Venezuela Los Chaguaramos, Caracas, 1050. Venezuela e-mail: ana.acevedo@ucv.ve / aacevedo1947@yahoo.com
ABSTRACT
Mutans Streptococci, in particular S. mutans and S. sobrinus, are generally considered to be the prime etiological bacteria of human dental caries. The aim of this study was to determine the frequency of mutans streptococci in dental plaque in three groups of caries-free and caries-affected Venezuelan children aged 2-6, 7-12, 13-19 years, and their possible association with dental caries. The frequency of mutans streptococci was determined in samples of pooled dental plaque collected from all detectable sources of 30 (62.5%) caries-affected and 18 (37.5%) cariesfree children. The samples were collected from all available tooth sites using a Hollenbak probe and immediately suspended in Ringer’s solution, serially diluted and cultured in Mitis Salivarius (MS) agar for total streptococci determination and Mitis Salivarius Bacitracin (MSB) for isolation of mutans streptococci. The bacterial identification procedure was done using the API Rapid Strep System. The criteria used to determine dmft and DMFT was established by Klein and Palmer (1941). Mean dmft and DMFT were 6.4±3.2 and 4.4±2.9, respectively. Ten (33%) out of 30 caries-affected children harbored mutans streptococci. The species most frequently found were S. mutans (20%), S. sobrinus (10%) and S. rattus (3.3%). Meanwhile, in the caries–free group only 6 out of 18 children (33%) harbored mutans streptococci, specifically S. mutans and S. sobrinus, both at 17%, with no significant difference between the two groups. These results indicate that the percentage of children that harbored mutans streptococci was similar in both groups, suggesting that other acidogenic species may be responsible for caries development.
Key words: Dental deposit; Mutans streptococci; Dental caries; Children.
RESUMEN
Presencia de Streptococcus Mutans en la placa dental proveniente de niños Venezolanos con caries y libres de caries
Los Streptococcus del “grupo” mutans en especial Streptococcus mutans y Streptococcus sobrinus han sido considerados como las principales bacterias responsables del desarrollo de la caries dental. El propósito principal de este estudio fue determinar la frecuencia de estreptococos del grupo mutans presentes en la placa dental de niños venezolanos divididos en tres grupos etarios, 2- 6, 7-12 y 13-19 anos, con caries y libres de caries y establecer una posible asociación con el desarrollo de caries dental. Muestras de placa dental de 30 (62,5%) niños con caries y 18 (37,5%) niños libres de caries fueron recolectadas de todas las superficies disponibles utilizando una espátula Hollenbak. Las muestras fueron recolectadas e inmediatamente suspendidas en solución Ringer, luego de lo cual se realizaron diluciones seriadas. Las muestras de placa dental fueron cultivadas en Agar Mitis Salivarius para la identificación de Streptococcus totales y en Agar Mitis Salivarius con Bacitracina para la determinación de Streptococcus del “grupo” mutans y posteriormente identificadas utilizando el sistema API Rapid Strep. El examen clinico para determinar el ceod y CPOD fue realizado siguiendo los criterios de Klein y Palmer (1941). El ceod y CPOD promedio fueron 6,4 ±3,2 y 4,4 ± 2,9, respectivamente. De los 30 niños con caries evaluados solo en 10 (33%) de estos se encontró Streptococcus del grupo mutans en la placa dental. Las especies del grupo mutans que se identificaron fueron S. mutans (20 %), S. sobrinus (10 %), y S. rattus (3,3%). En el grupo libre de caries el porcentaje de niños con Streptococcus del grupo mutans en su placa dental fue similar al grupo afectado por caries y las especies presentes fueron S. mutans (17%) y S. Sobrinus (17%). Estos resultados indican que el porcentaje de niños que presentaron Streptococcus del grupo mutans en su placa dental fue similar en los niños afectados por caries como en los libres de caries, lo que sugiere que otras especies acidogenicas de la cavidad bucal diferentes a las del grupo mutans parecieran estar jugando un papel importante en el desarrollo de caries dental.
Palabras claves: Caries dental; Niños; Placa dental; Streptococcus mutans.
INTRODUCTION
In Venezuela, two surveys of dental caries have been carried out, showing high caries prevalence1,2. Dental plaque is the biofilm associated to teeth, consisting of a microbial community in a matrix of polymer of bacterial and host origin that plays a primary role in the etiology of dental caries. Among the many bacterial species that have been identified in dental plaque, only a few are considered cariogenic3-5. Mutans Streptococci (MS) including S. mutans and S. sobrinus are well known as the group of oral microorganisms which have virulence factors related to cariogenicity. They possess a wide range of cariogenic traits which include a high rate of acid production, high acid tolerance, sucrose-mediated extracellular polysaccharide synthesis, and intracellular glycogen synthesis from a variety of dietary carbohydrates3,6,7 which are significant determinants of the cariogenicity of plaque.
For several decades, investigators have agreed on the fact that MS generally are implicated as the principal infectious bacteria responsible for dental caries in humans8-12, and are classified into seven species13. Of these, S. sobrinus and S. mutans are the most frequently isolated from the human oral cavity3. S. sobrinus is more cariogenic than S. mutans, nevertheless, it has been reported that strains of S. mutans and S. sobrinus isolated from infants and adolescents with varying caries experience did not show significant differences in aciduric and acidogenic activities14. S. mutans and S. sobrinus are associated more often with the more acidic response of caries-affected plaque and research has indicated that the coexistence of S. sobrinus and S. mutans is an important factor in the development of dental caries15, 16. Different studies have reported a correlation between plaque counts of MS and both caries prevalence and incidence3, 17-22. Hamada and Slade23, have shown that large numbers of S. sobrinus are found in saliva of individuals with high caries scores, as well as in plaque of caries-affected, but not caries-free mouths. Furthermore, it is reasonable to identify caries-susceptible individuals from the correlation between the presence of mutans streptococci and caries development24, 25. In contrast, other studies have reported that caries lesions can develop in the absence of S. mutans and their numbers are often low even when caries are present6-7, 26-28. In addition, supporting this statement, other investigations have found no correlation between the S. mutans count and the presence of caries29-32.
Based on the controversial results presented in literature, the aim of this study was to determine the frequency and distribution of Mutans Streptococci in dental plaque from caries-free and caries-affected Venezuelan children of different age groups and to associate their occurrence with the presence of dental caries.
PATIENTS AND METHODS
A total 48 children that attended the Department of Pediatric Dentistry of the Faculty of Dentistry, Central University of Venezuela and lived in the capital city (Caracas) were selected for the study. The patients were subsequently divided into three groups: Group A made up of 14 children aged 2 to 6 years, mean age 5.0±0.9 years; Group B of 17 children aged 7 to 12 years, mean age 8.5±1.6 years, and group C of 17 children aged 13 to 17 years, mean age 16.7±1.6 years. Each child was examined for caries status (dmft or DMFT) following the criteria established by Klein and Palmer33. Dental examination was carried out by the same calibrated examiner (MVR) using artificial light, probe # 5 and dental mirror # 23. The parents of all the children were asked to sign a consent form that allowed us to examine their children’s teeth and to take microbiological samples.
SAMPLING AND CULTURING
Two-hour fasting dental plaque samples were collected from each subject after clinical examination. After removal of saliva by a short blast of air, plaque was removed from all available tooth sites using a Hollenbak probe. Dental plaque was scraped from the available surfaces of the dentition in the morning (9 to 10 am) from each patient. The pool plaque sample was immediately placed in a sterile capped container, transported to the laboratory, weighed immediately and the wet weight was calculated by difference. The plaque was suspended in Ringer’s solution and homogenized by hand in a glass homogenizer. The suspension was serially diluted in ice-cold Ringer’s solution for microbial analysis to yield a final concentration of 5 mg wet weight plaque per mL.
BACTERIOLOGICAL MEDIA
Aliquots of 100μL of each appropriately serially diluted sample were plated in duplicate on Brain- Heart-Infusion (BHI) for total microorganism count, as well as in ready-poured Mitis Salivarius Agar media with tellurite (0.1%) (Difco Labs. Detroit, Ml) for total streptococci count and Mitis-Salivarius Bacitracin (MSB) agar (0.2 units/ mL) for mutans streptococci. Acidogenic ratio determinations34 were carried out on bromocresol purple agar. The media contained glucose 12 g; K2HPO4 1.2 g; KH2PO4 1.2 g; tryptone 5 g; yeast extract 5 g; agar 15 g; bromocresol purple 16 ml of a 0.2% sterile stock solution. The pH was adjusted to 7.4 with KOH. After inoculation, all media were incubated anaerobically in jars (Gas Pack System) at 37oC for 48 h.
BACTERIAL COUNT AND ISOLATION
Each colony was counted for total Streptococci on MS agar and a representative colony of each distinct colonial morphology from MSB was selected and grown overnight in 5 ml of pre-sterilized Todd- Hewitt broth at 37oC. Each colony isolated was then identified biochemically using the API Rapid Strep System (API SYSTEMS S.A.). The results were recorded after 24 h of isolation and tests showing unclear or weak results were re-checked using biochemical tests (fermentation of manitol, sorbitol, and enzymatic tests such as catalase activity). Acidogenic organisms were recognized by the color change from purple to yellow and the acidogenic ratio was calculated.
RESULTS
Sample distribution according to caries experience expressed as a percentage is illustrated in Table 1. Children under study were divided into three groups, according to age: Group A – children aged 2 - 6 years, of whom 57% were caries-affected and 42% were caries-free; in group B, 76% and 24% of the subjects were caries-affected and caries-free respectively; and group C was made up of children aged 13 to 17 years, of whom 53% were cariesaffected and 47% caries-free. Table 2 shows the mean dmft and DMFT indexes in each age group. A high dmft was found in the 2 to 6-year-old children. The children belonging to the 7 to 12-year-old age group showed a higher but non-significant DMFT index compared to the group of 13 to 19-year-olds. The total mean DMFT for the caries-affected children was 4.4± 2.2.
Table 1: Sample distribution according to caries experience expressed as a percentage
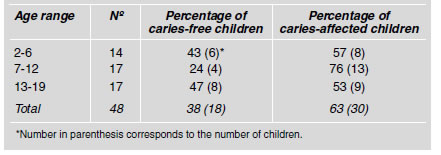
Table 2: Mean dmft and DMFT in caries-affected children
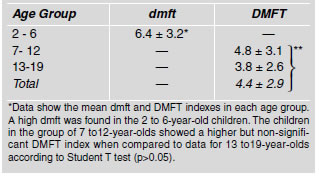
The mean values for total streptococci count (CFU) in dental plaque samples from children in different age groups as well as from caries-free and caries-affected children are shown in Table 3. The mean total values of streptococci counts present in the plaque from children aged 2-6, 7-12 and 13-19 years were not statistically different (p>0.05). In caries-free and caries-affected children, the total streptococci CFU was similar for two age groups, except in the group of 7 to 12- year-old caries-free children, which showed a higher number of bacterial counts. However when these values were analyzed, no statistical difference was found (p>0.05). Similarly, when the total CFU counts in plaque for the three age groups were compared, no significant difference was observed. Since the total number of streptococci in caries-affected and caries-free children was not different, we decided to analyze the distribution of mutant streptococci (MS) species in dental plaque samples of the children in the different age groups. The percentages of caries- affected and caries-free subjects that harbored mutant streptococci species are shown in Table 4. The results from the children of all ages showed that caries-free children harbored S. mutans, (17%) and S. sobrinus (17%) but not S. rattus.
Table 3: Mean values of total streptococci count in dental plaque samples of caries-free and caries-affected children expressed as CFUx105/mg wet weight ¹.
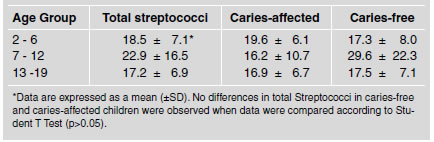
Table 4: Distribution of Mutans streptococci (MS) species in dental plaque samples from caries-free and caries-affected children.
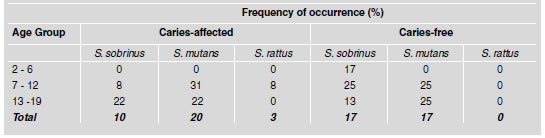
On the other hand, 10% of the caries-affected children showed the presence of S. sobrinus, 20% showed S. mutans and 3% showed S. rattus. In the group 2 to 6-year-old children affected by caries, none of the MS species analyzed was detected, although S. sobrinus was found in the caries-free subjects. A higher percentage with MS was found in the 7 to 12-year-old group (Table 4). It is noteworthy that the percentage of children with S. mutans in the caries-affected group was slightly higher than in the caries-free subjects, but the percentage of children with S. sobrinus in the caries-free group was significantly higher than in the caries-affected subjects (p<0.05). The presence of S. rattus was detected in 8% of the caries-affected subjects. It is noticeable that S. rattus was not present in any of the caries-free children. On the other hand, the results obtained from caries-free and caries-affected children grouped in the 13 to 19-year-old age range showed that the percentage of individuals with S. mutans count was similar (25 and 22% respectively), S. rattus was not found, and the percentage of children that harbored S. sobrinus was higher but not significant in cariesaffected subjects.
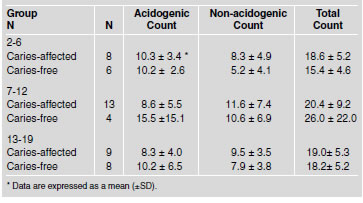
Table 5 shows the mean values of total, acidogenic and non-acidogenic bacterial count in dental plaque expressed in CFU x105/mg wet weight-1. Caries-free and caries-affected children 2-6 years old showed a similar number of acidogenic bacteria (10.2±2.6 and 10.3±3.4 respectively). However, non-acidogenic bacteria were slightly higher, though not significantly, in the caries-affected group as compared to the caries-free children (8.3±4.9 and 5.2±4.1 respectively). The total number of CFU was lower in the caries-free group than in the caries-affected. However the differences observed in all bacterial types were not statistically significant (p>0.05). A similar pattern was found in the 7 to 12 and 13 to 19-year-old children when the acidogenic, non-acidogenic and total counts were analyzed. When the mean total number of acidogenic, non-acidogenic and acidogenic plus non-acidogenic (total) bacterial counts were compared in the three age groups, the differences were also not statistically significant (p>0.05).
Table 5: Mean total count values, acidogenic and non-acidogenic in dental plaque samples from children in each group studied (expressed in CFUx105/mg wet weight ¹).
DISCUSSION
Mutans streptococci (MS) have been considered the main etiological agents in caries development3,13. Later on, van Houte35,36 suggested the importance of streptococci other than MS in the initiation of dental caries; however, even today there are conflicting results on this subject. In this investigation we evaluate the total streptococci count, the distribution of S. mutans, S. sobrinus and S. rattus in dental plaque from caries-affected and cariesfree children, since it has been shown that the coexistence of S. sobrinus and S. mutans is an important factor in the development of dental caries36 as well as the total acidogenic and non-acidogenic count as a parameter to be associated with caries occurrence. The estimation of the prevalence of mutans streptococci was based on the condition that each subject was considered as the basic unit influencing the prevalence of these bacteria from individual sites. All comparisons between mutans scores were therefore first made within each individual and then statistically analyzed for all subjects.
The present study has confirmed the patterns observed in other studies for the distribution of mutans streptococci in caries-affected and caries-free children and that local variations exist in the prevalence of cariogenic species within dental plaque. S mutans and S. sobrinus were nevertheless found on apparently healthy sites, which has been seen in other studies previously reported by Babaahmedy et al.16. Several reports have demonstrated that MS are isolated more frequently as the child grows older37-39. Moreover, many authors have reported that children commonly acquire mutans streptococci between the 1st and 3rd year of life10,40. In this investigation mutans streptococci species were not detected in plaque from 2 to 6-yearold, caries-free or caries-prone children, except for S. sobrinus, which was found in 1 child in the caries-free group. This result suggests a delay in the colonization of mutant streptococci in the dental plaque of this group of children. However, the high caries rate observed in the caries-affected children may suggest that Streptococcus, in addition to species such as lactobacilli and bifidobacterium41, could be involved in the development of dental caries. On the other hand, all the cariesfree and caries-prone subjects in the 7 to 12 and 13 to 19-year-old groups harbored both S. mutans and S. sobrinus in their dental plaque. Moreover, the percentage of children with S. sobrinus in the caries-free subjects was significantly higher than in the caries-affected group (Table 4). However, the percentage of children with S. mutans in the caries-free group was lower than in the caries-affected group. This result suggests that a higher number of S. mutans associated to S. sobrinus may be needed to increase the risk of development of dental caries. In addition, Lang et al.17 suggested that local factors such as differences in exposure of saliva or variations of fluoride levels42 could be implicated in the association of mutans streptococci with healthy sites.
The role of acid-producing bacteria in the carious process has been reviewed by different authors6, 17,43. Stephan44 was the first to show that the plaque pH can fall below 5.0 after a sugar rinse and it is reasonable to predict from the acidogenic theory of caries that plaque samples from caries-affected individuals will have a larger proportion of acidogenic organisms than similar samples from caries-free subjects. However, the results of this investigation indicate that the proportions of acidogenic to nonacidogenic in dental plaque from caries-affected and caries-free children were similar (Table 5). This indicates that factors other than the microbiological are determinant in the initiation of the caries process. Controversial results have been reported in relation to the association between the presence of mutans streptococci and dental caries45,46. In this study, no association was observed to support the idea that caries could occur in the absence of mutans streptococci47. The results of this investigation indicate that the microbiological analysis of dental plaque could be a useful tool to identify the caries risk at individual or community level, as well as to improve diagnosis and treatment.
ACKNOWLEDGMENTS
We wish to thank the parents and children who took part in this investigation. This work was supported by the Faculty of Dentistry, Central University of Venezuela.
1. Cova Rey, Lozada I. Estudio para la planificacion integral de la Odontologia en Venezuela. Area de Salud Oral. Ministerio de Sanidad y Asistencia Social, Departamento de Odontologia Sanitaria, Venezuela 1972. [ Links ]
2. Rivera L, Acevedo A, Nunez A. National survey of dental caries prevalence in 6-8, 12 and 15 year old children in Venezuela. Final report. WHO/PAHO, 1998. [ Links ]
3. Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiol Rev 1986;50:353-380. [ Links ]
4. Tanzer JM. Microbiology of dental caries. p. 377-424. In: J. Slots and M. Taubman (ed.), Contemporary Oral Microbiology and Immunology. Mosby Yearbook, St. Louis, USA 1992. [ Links ]
5. Thenisch NL, Bachmann LM, Imfeld T, Leisebach Zinder, Steurer J. Are mutans streptococci detected in preschool children a reliable predictive factor for dental caries risk. A systematic review. Caries Res 2006;40:366-374. [ Links ]
6. van Houte J. Role of microorganisms in caries etiology. J Dent Res 1994a;73:672-681. [ Links ]
7. van Houte J, Lopman J, Kent R. The predominant cultivable flora of sound and carious human root surfaces. J Dent Res 1994b;73:1727-1734. [ Links ]
8. Gibbons RJ, van Houte J. Dental Caries. Ann Rev Med 1975;26:121-136. [ Links ]
9. van Houte J. Bacterial specificity in the etiology of dental caries. Int Dent J. Dec. 1980;30:305-326. [ Links ]
10. Caufield PW, Cutter GR, Dasanayaka AP. Initial acquisition of mutans streptococci by infants: evidence for a discrete window of infectivity. J Dent Res 1993;72:37-45. [ Links ]
11. Milgrom P, Ly KA, Roberts MC, Rothen M, Mueller G, Yamaguchi DK. Mutans streptococci dose response to xylitol chewing gum. J Dent Res 2006;85:177-181. [ Links ]
12. Guo LH, Shi JN, Zhang Y, Liu XD, Duan J, Wei S. Oral Microbiol Immunol 2006:21:372-380. [ Links ]
13. Whiley RA, Russel RRB, Hardy JM, Beighton D. Streptococcus downei sp. nov. for strain previous described as Streptoccus mutans serotype h. Int J Syst Bacteriol 1988;38:25-29. [ Links ]
14. Tanzer JM. On Changing the Cariogenic Chemistry of Coronal Plaque. J Dent Res 1989;68:1576-1587. [ Links ]
15. Lindquist B, Emilson CG. Dental location of Streptococcus mutans and Streptococccus sobrinus in human harbouring boyh species. Caries Res 1991;25:146-152. [ Links ]
16. Babaahmedy, Challacombe, Marsh, Newman. Ecological study of Streptococcus mutans, Streptococcus sobrinus and Lactobacillus spp. At subsites from approximal dental plaque from children. Caries Res 1998;32:51-58. [ Links ]
17. Lang, NP, Hotz PR, Gusberti FA, Joss A. Longitudinal clinical and microbiological study on the relationship between infection with Streptococcus mutans and the development of caries in humans. Oral Microbiol Immunol 1987;2:39-47. [ Links ]
18. Crossner CG, Claesson R, Johansson T. Presence of mutans streptococci and various types of lactobacilli in interdental spaces related to development of proximal carious lesions. Scand J Dent Res 1989;97:307-315. [ Links ]
19. Beighton D, Manji F, Baelum V, Fejerskov O, Johnson NW, Wilton JM. Associations between salivary levels of Streptococcus mutans, Streptococcus sobrinus, lactobacilli, and caries experience in Kenya adolescents. J Dent Res 1989;68:1242-1246. [ Links ]
20. de Soet JJ, Holbrook WP, van Amerongen WE, Schipper E, Homburg CHE, de Graaff J. Prevalence of Streptococcus sobrinus in relation to dental caries in children from Iceland and the Netherlands. J Dent Child 1990;57:337-342. [ Links ]
21. Sigurjons H, Magnusdottir MO, Holbrook WP. Cariogenic bacteria in a longitudinal study of approximal caries. Caries Res 1995;29:42-45. [ Links ]
22. Mattos-Graner RO, Correa MS, Latorre MR, Peres RC, Mayer MP. Mutans streptococci oral colonization in 12-30- month-old Brazilian children over a one-year follow-up period. J Public Health Dent 2001;61:161-167. [ Links ]
23. Hamada, S. and Slade, HD. Biology, Immunology and Cariogenicity of Streptococcus mutans, Microbial Rev 1980; 44:331-384. [ Links ]
24. Brathall D. Selection for prevention of high caries risk groups. J Dent Res 1980;59:2178-2182. [ Links ]
25. Zickert I, Emilson CG, Krasse B. Effect of caries preventive measures in children highly infected with the bacterium Streptococcus mutans. Arch Oral Biol 1982;27:861-868. [ Links ]
26. Sims S. Streptococcus mutans and vaccines for dental caries: a personal commentary and critique. Community Dent Health 1985;2(2):129-147. [ Links ]
27. Determinants of virulence in dental plaque. In: Cariology for the ninenties. van Houte J Bowen WH, Tabak LA, 1993 editors Rochester, NY: University of Rochester Press. [ Links ]
28. van Houte J, Lopman J, Kent R. The final pH of bacteria comprising the predominant flora on sound and carious human root and enamel surfaces. J Dent Res 1996;75:1008-1014. [ Links ]
29. Hardie JM, Thompson PL, South RJ, Marsh PD, Bowden GH, McKee AS, Fillery ED, Slack GL. A longitudinal epidemiology study on dental plaque and development of dental caries: Interium results after two years. J Dent Res 1977; 55:C90-C98. [ Links ]
30. Carlsson J, Kujala U, Edlund MB. Pyruvate dehydrogenase activity in Streptococcus mutans. Infect Immun 1985; 49:674-678. [ Links ]
31. Marsh PD, Bevis RA, Newman HN, Hallsworth AS, Robinson C, Weatherell JA, Pitter AF. Antibacterial activity of some plaque- disclosing agents and dyes. Caries Res 1989; 23:348-350. [ Links ]
32. Macpherson LMD, MacFarlane TW, Geddes DAM, Stephen KW. Assessment of the cariogenic potential of Streptococcus mutans strain and its relationship in vivo caries experience. Oral Microbial Immunol 1992;7:142-147. [ Links ]
33. Klein H, Palmer LE. Studies in dental caries I. Dental status and dental needs of elementary school children. Public Health Rep 1941;53:751-765. [ Links ]
34. Handelman SL, Mills JR, Meggo I. Medium for differentiating acidogenic bacteria. Arch Oral Biol 1968;13:1187-1196. [ Links ]
35. van Houte J, Sansone C, Joshipura K, Kent R. Mutans streptococci and non-mutans streptococci acidogenic at low pH, and in vitro acidogenic potential of dental plaque in two different areas of the human dentition. J Dent Res. 1991;70:1503-1507. [ Links ]
36. van Houte J, Sansone C, Joshipura K, Kent R. In vitro acidogenic potential and mutans streptococci of human smooth-surface plaque associated with initial caries lesions and sound enamel. J Dent Res 1991;70:1497-1502. [ Links ]
37. Alaluusua S, Renkonen O-V. Streptococcus mutans establishment and dental caries experience in children from 2 to 4 years old. Scand J Dent Res 1983;91:453-457. [ Links ]
38. Catalanotto FA, Shklair IL, Keene HJ. Prevalence and localization of Streptococcus mutans in infants and children. J Am Dent Assoc 1975;91:606-609. [ Links ]
39. Fujiwara T, Sasada E, Mima N, Oshima T. Caries prevalence and salivary mutans streptococci in 0-2 year old children of Japan. Community Dent Oral Epidemiol 1991;19;151-154. [ Links ]
40. Wendt IK, Hallonstenai Koch Birkhed D. Analysis of caries-related factors in infant and toddlers living in Sweden. Acta Odontol Scan 1996;54:131-137. [ Links ]
41. Crociani F, Biavati B, Alessandrini A, Chiarini C, Scardovi V. Bifidobacterium inopinatum sp.nov. and Bifidobacterium denticolens sp.nov. Two new species isolated from human dental caries. International Journal of Systematic Bacteriology 1996;46:564-571. [ Links ]
42. Kaneko N, Yoshihara A, Ida H, Nomura Y, Imai S, Nisisawa T, Sakuma S, Hanada N, Miyasaki H. Influence of a fluoride mouth rinse on mutant streptococci in school children. Caries Res 2006;40:501-507. [ Links ]
43. Kleinberg I. A Mixed-Bacteria Ecological Approach to Understanding the Role of the Oral Bacteria In Dental Caries Causation: An Alternative to Streptococcus mutans and the Specific Plaque Hypothesis. Critical Rev in Oral Biol 2002; 13 (Issue 2):108-125. [ Links ]
44. Stephan RM. Intra-oral hydrogen-ion concentrations associated with dental caries activity. J Am Dent Assoc 1944; 23:257-266. [ Links ]
45. Effect of saliva and salivary factors on the metabolism of the mixed oral flora. In: Microbial Aspects of Dental Caries. Kleinberg I, Kanapa JA and Craw D. 1976. Edited by Stiles H.M., Loesche WJ and O’Brien TC. Information Retrieval, New York.
46. Kleinberg I, Kanapa JA, Chatterjee R, Craw D, D’Angelo N, Sandham HJ. Metabolism of nitrogen by the oral mixed bacteria. In: Saliva and Dental Caries (edited by Kleinberg I, Ellison SA and Mandel ID) pp. 357-378. Information Retrieval, New York 1979.
47. Loeshe WJ, Straffon LH. Longitudinal investigation of the role of Streptococcus mutans in human fissure decay. Infect Immun 1979;26:498-507. [ Links ]














