Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Acta Odontológica Latinoamericana
versión On-line ISSN 1852-4834
Acta odontol. latinoam. vol.23 no.3 Buenos Aires dic. 2010
ARTÍCULOS ORIGINALES
Evaluation of vascular endothelial growth factor (VEGF) in interradicular bone marrow in olpadronate treated animals
María M. Pujadas Bigi, Natalia D. Escudero, Ángela M. Ubios, Patricia M. Mandalunis
Department of Histology and Embryology. Faculty of Dentistry. University of Buenos Aires. Argentina.
CORRESPONDENCE Dra.Maria Montserrat Pujadas Bigi Department of Histology and Embryology M.T. de Alvear 2142, 1o “A” C1122AAH Ciudad Autonoma de Buenos Aires Argentina E-mail: montsepujadas@gmail.com
ABSTRACT
Vascular endothelial growth factor (VEGF) is a protein that increases vascular permeability and induces the proliferation, migration and survival of endothelial cells. Bisphosphonates (BPs) are antiresorptive drugs that are widely used in the treatment of bone metabolism diseases and bone metastases. Since 2003, cases of bisphosphonate-related osteonecrosis of the jaw (BRONJ) have been reported. Few papers explain the mechanisms that induce BRONJ; some authors mention alterations in bone remodelling and a certain antiangiogenic effect of BPs. The aim of this study is to evaluate the expression of VEGF in bone marrow cells and the number of blood vessels and area occupied by them in animals treated with the BP sodium olpadronate (OPD). We used 16 Wistar rats, 60 days old, divided into two groups, experimental (OPD) and control. The OPD group received 0.3 mg/kg/week intraperitoneal OPD for 5 weeks. The control group received an equivalent intraperitoneal volume of physiological saline solution. After euthanasia, hemimandibles were processed and mesio-distal histological sections of the first molar were prepared. Sections were stained with hematoxylin-eosin (HE), immunohistochemical detection of VEGF was performed (sc- 7269) and the following histomorphometric parameters were evaluated: In HE-stained sections - number of blood vessels per sq. mm. and percentage (%) of area occupied by blood vessels in relation to total area evaluated; in sections with immunohistochemical detection of VEGF – number of VEGF+ bone marrow cells per sq. mm. Data underwent statistical analysis. Number of blood vessels/mm2 was significantly lower in the OPD group (OPD: 92 ± 16; Control: 140 ± 31; p<0.05) and % vascular area/ total area evaluated showed no significant difference (OPD: 15.6 ± 6.1; Control: 10.2 ± 4.2). Number of VEGF+ cells/mm2 was lower in the OPD group than in the control group, with statistically significant differences (OPD: 7804.8 ± 597; Control: 13187.6 ± 894; p<0.001). The results of this study suggest that monosodium olpadronate has an antiangiogenic effect. Further studies are needed to reveal its potential as an antitumor agent and its connection with the onset of BRONJ.
Key words: VEGF; Bone marrow; Blood vessels; Anti-angiogenic agent.
RESUMEN
Evaluación del VEGF en la medula ósea del hueso interradicular en animales tratados con olpadronato
El factor de crecimiento vascular (VEGF) es una proteina que incrementa la permeabilidad vascular, induce la proliferacion, migracion y supervivencia de las celulas endoteliales. Los bifosfonatos (BFs) son drogas antirresortivas ampliamente utilizadas en el tratamiento de enfermedades que alteran el metabolismo oseo y de metastasis oseas. Desde el 2003 se han reportado casos de osteonecrosis de maxilar asociada al uso de BFs (ONAB). Escasas publicaciones explican los mecanismos que inducen la ONAB, algunos autores mencionan las alteraciones en la remodelacion osea y un cierto efecto antiangiogenico de los BFs. El objetivo del presente trabajo es evaluar la expresion de VEGF en celulas de la medula osea y el numero y el area ocupada por vasos sanguineos en animales tratados con el BF olpadronato monosodico (OPD). Se utilizaron 16 ratas Wistar de 60 dias divididas en dos grupos, experimental (OPD) y control. El grupo OPD, recibio 0,3 mg/kg/sem de OPD via IP, durante 5 semanas. El grupo control, recibio un volumen equivalente via IP de solucion fisiologica. Luego de practicada la eutanasia se obtuvieron las hemimandibulas y se procesaron para obtener cortes histologicos mesio-distales del primer molar. Se realizo la coloracion hematoxilina-eosina (HE) y la deteccion inmunohistoquimica de VEGF (sc-7269) y se evaluaron los siguientes parametros histomorfometricos: En cortes con H&E: Numero de vasos sanguineos por mm2 y porcentaje (%) de area ocupada por los vasos sanguineos en relacion al area total evaluada; en cortes con la deteccion inmunohistoquimica de VEGF: Numero de celulas medulares VEGF+ por mm2. Los datos fueron estadisticamente analizados. El N° vasos sanguineos/mm2 fue significativamente menor en el grupo OPD (OPD: 92 ± 16; control: 140 ± 31; p<0,05) y el % area vascular/area total evaluada no mostro diferencias significativas (OPD: 15,6 ± 6.1; Control: 10.2 ± 4.2). El N° de celulas VEGF+/mm2 en el grupo OPD fue menor que en el grupo control con diferencias estadisticamente significativas (OPD: 7804,8 ± 597; Control: 13187,6 ± 894; p<0,001). Los resultados de este estudio sugieren que el olpadronato monosodico tiene un efecto antiangiogenico. Futuros estudios revelaran su potencial como agente antitumoral asi como tambien su relacion con la aparicion de ONAB.
Palabras clave: VEGF; Medula osea; Vasos sanguineos; Agente antiangiogenico.
INTRODUCTION
Vascular endothelial growth factor (VEGF) was first isolated simultaneously by Ferrara and Henzel 1 and Plouet et al.2, who published the purification of an NH2-terminal amino acid sequence of a mitogen specific for endothelial cells, which they called, respectively, vascular endothelial growth factor (VEGF) and vasculotropin (VPF). It was subsequently shown that the activity of both factors corresponded to the same molecule. The discovery that VEGF was powerful, specific and freely diffusible for vascular endothelial cells led to the hypothesis that it played a unique part in regulating physiological and pathological angiogenesis3. VEGF is a mitogen that is specific for endothelial cells and has no mitogenic activity on any other kind of cell.4 In three-dimensional in vitro models it promotes angiogenesis by inducing the formation of capillarylike structures in a collagen gel5, and in in vivomodels it induces a strong angiogenic response2,6. In endothelial cell cultures it works as a survival factor7,8, and both in vivo and in vitro, it induces the appearance of fenestrations in the vascular endothelium9.
Bisphosphonates are analogues of pyrophosphates, in which the linking oxygen is replaced by a carbon atom with different side chains. They were used industrially in the early 19th century as anti-scaling and anticorrosive agents. It was later discovered that they could control the dissolution of calcium and phosphate in vitro, as well as bone mineralization and resorption in vivo10. Later on, these drugs were developed for the treatment of various diseases that alter bone metabolism, such as osteoporosis, Paget’s disease, hypercalcaemia of malignancy and bone metastases11. They may be administered orally or intravenously, and their potency varies according to whether or not they are nitrogenated and according to the side-chains they contain. For about the last decade, BPs (particularly pamidronate and zoledronic acid) have been used as therapeutic agents for bone metastases of prostate cancer. They reduce the symptoms and bone complications because they inhibit the osteoclast activity that leads to bone destruction12. Current pre-clinical trials have shown that BPs can also reduce visceral metastases; however, the anti-tumor action mechanism on non-bone metastases is as yet unknown13.
As of 2003, reports have been published on patients with bisphosphonate-related osteonecrosis of the jaw (BRONJ)14,15, related to either intravenous IV16,17 or oral18,19 administration of bisphosphonates. Some authors assign the onset of BRONJ to the effect of BPs on bone metabolism, bone marrow cells and an antiangiogenic effect20-22. However, the studies showing association between the onset of BRONJ and the antiangiogenic effect of BPs are few and controversial. Olpadronate (OPD) is a monosodium amino-bisphosphonate of similar potency to alendronate, with excellent gastric tolerance thanks to its double methylation23. Although many studies describe the anti-resorption effect of OPD, there is no study describing an antiangiogenic effect nor its antitumor capacity or its association with BRONJ. The aim of this study is to assess the antiangiogenic effect of OPD by evaluating the expression of VEGF and the number and surface area of blood vessels in the bone marrow of the interradicular bone of the first lower molar.
MATERIALS AND METHODS
Sixteen Wistar rats 60 days old were divided into two groups: experimental (OPD) and control (C). The OPD group received 0.3 mg/kg/week OPD intraperitoneally (IP) for five weeks, and the sh group received an equivalent volume of physiological saline solution, IP. The rats were euthanized in the sixth week after the beginning of the experiment. They were sedated with 0.5 mg/kg acepromazine after which they were given a lethal overdose of sodium thiopental (PentotetR, Richmond Vet, Buenos Aires, AR). The hemimandibles were resected and fixed in 10% PBS formalin buffer. Then they were de-calcified in 10% EDTA (ethylene diamine tetraacetic acid) and processed for embedding in paraffin. Histological sections were made of the first lower molar, oriented mesio-distally. Two sections were made per experimental unit: one for H&E staining and the other for immunohistochemical detection of VEGF (sc-7269). Digital photographs were taken of all sections with an original magnification of 400X, using Image Pro Plus 4.0 software. Histomorphometric evaluation was performed on a standardized area of the interradicular bone. The area was standardized in the most apical third of the interradicular bone of the first lower molar (Fig. 1). The following histomorphometric parameters were evaluated in the bone marrow: a) in the sections stained with H&E: number of blood vessels per square millimeter (n° blood vessels/mm2) and percentage of area occupied by blood vessels compared to total area evaluated (% vascular area/total area); b) in the sections with immunohistochemical detection of VEGF, the number of VEGF+ marrow cells per square millimeter (n° VEGF+ cells/mm2) was evaluated. Statistical analysis was performed with Student’s T test. All procedures were reviewed and approved by the Ethics Committee of the School of Dentistry, which follows the Guide for the Care and Use of Laboratory Animals (NRC 1996).
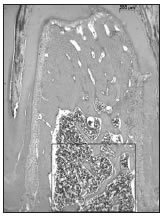
Fig. 1: Microphotograph of interradicular bone of the first lower molar, oriented mesiodistally, showing the area used for the histomorphometric measurements.
RESULTS
The densitometry of the apical third of the interradicular bone indicated 20% trabeculae and 80% bone marrow in both the experimental group and the control group. The number of blood vessels per square millimeter was significantly lower in the OPD group; OPD: 92 ± 16, C: 140 ± 31; p<0,05 (Fig. 2), while the percentage of area occupied by blood vessels showed no significant difference between groups; OPD: 15.6 ± 6.1; C: 10.2 ± 4.2 (Fig. 3). Images are provided in Figures 4a (control) and 4b (OPD). The number of VEGF+ cells per square millimeter in the OPD group was significantly lower than in the control group; OPD: 7804.8 ± 597; control: 13187.6 ± 894; p<0,001 (Fig. 5).
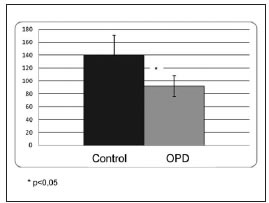
Fig. 2: Graph showing number of blood vessels/mm2. Note that it is significantly lower in the OPD group than in the control group (p<0.05).
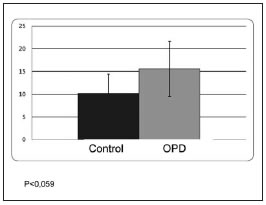
Fig. 3: Graph showing percentage of area occupied by blood vessels in the groups studied. The difference was not statistically significant.
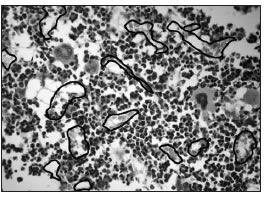
Fig. 4a: Microphotographs of sections stained with H&E, where the border of the blood vessels has been marked. a:control.
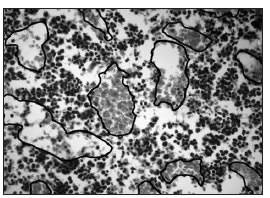
Fig. 4b: OPD. The number of vessels is lower and the area occupied by the vessels is greater in the OPD group than in the control group.
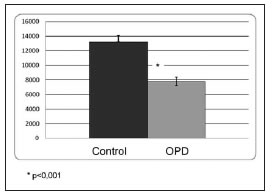
Fig. 5: Number of VEGF(+) cells per Squire millimeter in the groups studied. Note that it is lower in the OPD group than in the control group, with statistically significant differences at p<0.001.
DISCUSSION
The results of this study showed that olpadronate bisphosphonate administered intraperitoneally reduced the number of blood vessels/mm2 compared to the control group, while no difference was found in the percentage of area occupied by blood vessels between the experimental group and the control group. The number of VEGF+ marrow cells was significantly lower in the experimental group than in the control group. The fact that there were fewer blood vessels, while at the same time there was no significant difference in the area occupied by the blood vessels between groups, might be a functional adaptation of vascularization to tissue requirements by means of an increase in the vascular area in experimental animals. The reduction of VEGF+ cells suggests an antiangiogenic effect of olpadronate, not described to date in the literature. Although several papers have described the antitumor action of certain bisphosphonates, there is still controversy regarding the existence of antiangiogenic action mediated by a reduction in VEGF.
The antitumor action of powerful bisphosphonates such as zoledronic acid24 and minodronic acid (YM529)25 is related to the prevention of proliferation and induction of apoptosis, and they are believed to act synergically with chemotherapeutic agents; yet none of these reports mention changes in the expression of VEGF. In a clinical study conducted by Amir et al. 200926 on patients medicated with zoledronic acid, the concentration of VEGF was measured in platelets, showing no significant change compared to patients who did not undergo this treatment; whereas Lipton27, in the same year, performed a clinical study on patients with bone metastases who were under treatment with the BP zoledronic acid, and found that basal levels of VEGF were significantly reduced, suggesting that zoledronic acid might have clinically relevant antiangiogenic properties. Hashimoto et al. 200728 report that alendronate has an antiangiogenic effect by inhibiting the generylgeranylation of Rho without altering the expression of VEGF.
The study of the antiangiogenic effect of bisphosphonates began to increase in relevance in 2003, when bisphosphonate-associated osteonecrosis of the jaws (BRONJ)14,15, was reported and partly attributed to the effect of BPs on angiogenesis, bone remodeling and bone marrow cells. Despite the fact that many papers have been published, the antitumor and physiopathology of BRONJ is still under study. The antiangiogenic action produced by BPs in the bone marrow of animals free of disease, plus the inhibitory action on bone metabolism, particularly at the expense of bone resorption, may partly explain the physiopathology of BRONJ and the antitumor effect of these drugs.
CONCLUSION
The results of this study suggest that monosodium olpadronate has an antiangiogenic effect. As it is a relatively new bisphosphonate that is still under study, further research is needed to reveal its potential as an antitumor agent and its connection to the onset of BRONJ.
ACKNOWLEDGEMENTS
Grants: UBACyT O 018 and UBACYT O 406, University of Buenos Aires. The authors want to express their gratitude to Mariela Lacave, Carolina Acuňa and Vet Marianela Lewicki and Carolina Roman for their technical assistance.
1. Ferrara N. Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun.1989 Jun 15;161:851-8. [ Links ]
2. Plouet J. Schilling J. Gospodarowicz D. Isolation and characterization of a newly identified endothelial cell mitogen produced by AtT-20 cells. EMBO J. 1989 Dec 1;8:3801-6.
3. Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J Mol Med. 1999;77:527-543.
4. Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997 Feb;18:4-25.
5. Pepper MS, Ferrara N, Orci L, Montesano R. Potent synergism between vascular endothelial growth factor and basic fibroblast growth factor in the induction of angiogenesis in vitro. Biochem Biophys Res Commun 1992; 189:824-831.
6. Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989 Dec 8;246:1306-9.
7. Gerber HP, Dixit V, Ferrara N. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl- 2 and A1 in vascular endothelial cells. J Biol Chem. 1998 May 22;273:13313-6.
8. Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3’-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998 Nov 13;273:30336-43.
9. Roberts WG, Palade GE. Neovasculature induced by vascular endothelial growth factor is fenestrated. Cancer Res. 1997 Feb 15;57:765-72.
10. Rodan GA, Fleisch HA. Bisphosphonates: Mechanisms of Action. J Clin Invest. 1996 June 15:97:2692-96. 11. Fleisch HA. Biphosphonates in osteoporosis. Eur Spine J. 2003;12 Suppl. 2:142–146.
12. Coleman RE. Metastasic bone disease: clinical features, pathophysiology and treatment strategies. Ann Oncol 2005; 16:687-695.
13. Park IH, Ro Jungsil, Nam BH, Kwon Y, Lee KS. Potential antitumor effects of nitrogen-containing bisphosphonate in hormone receptor negative breast cancer patients with bone metastases. BMC Cancer 2009;9:1-9.
14. Marks RE. Pamidronate (Aredia) and Zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61:1115-18.
15. Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bipshosphonates: a review of 63 cases. J Oral Maxillofac Surg. 2004;62:527-534.
16. Bamias A, Kastritis E, Bamia C, Moulopoulos LA, Melakopoulos I, Bozas G, Koutsoukou V, Gika D, Anagnostopoulos A, Papadimitrou C, Terpos E, Dimopoulos MA. Osteonecrosis of the jaws in cancer after treatment with bisphosphonates: Incidence and risk factors. J Clin Oncol. 2005;23:8580-8587.
17. Marx RE, Sawatari Y, Fortin M, Broumand V. Biphosphonate- Induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention and treatment. J Oral Maxillofac Surg. 2005;63:1567-1575.
18. Ruggiero S.L. et al. American Association of Oral and Maxillofacial Surgeons Position Paper on Biphosphonate-related osteonecrosis of the Jaws – 2009 Update. Aust Endod J. 2009;35:119-130.
19. Yoneda T, Hagino H, Sugimoto T, Ohta H, Takahashi S, Soen S, Taguchi A, Toyosawa S, Nagata T, Urade M. Biphosphonate-related osteonecrosis of the jaws: position paper from the Allied Task Force Committee of Japanese Society for Bone and Mineral Research, Japan Osteoporosis Society, Japanese Society of Periodontology, Japanese Society for Oral and Maxillofacial Radiology and Japanese Society of Oral and Maxillofacial Surgeons. J Bone Miner Metab. 2010;28:365-383.
20. Garcia Saenz JA, Lopez Tarruella S, Garcia Paredes B, Rodriguez Lajusticia L, Villalobos L, Diaz Rubio E. Osteonecrosis of the jaw as an adverse biphosphonate event: Three cases of bone metastatic prostate cancer patients treated with zoledronic acid. Med Oral Patol Oral Cir Bucal. 2007;12:E351-6.
21. Van den Wyngaert T, Huizing MT, Vermorken JB. Osteonecrosis of the jaw related to the use of bisphosphonates. Curr Opin Oncol. 2007;19:315-322.
22. Allen MR, Burr DB. The pathogenesis of biphosphonaterelated osteonecrosis of the jaw: so many hypotheses, so few data. J Oral Maxillofac Surg. 2009;67:61-70.
23. Escudero ND, Lacave M, Ubios AM, Mandalunis PM. Effect of monosodium olpadronate on osteoclasts and megakaryocytes: an in vivo study. J Musculoskelet Neuronal Interact. 2009;9:109-20.
24. Neville-Webbe HL, Gnant M, Coleman RE. Potential anticancer properties of bisphosphonates. Semin Oncol. 2010; 37:S53-65.
25. Yuasa SK, Nogawa M, Kimura S, Segawa H, Maekawa T. A third-generation bisphosphonate, minodronic acid (YM529) successfully prevented the growth of bladder cancer in vitro and in vivo. Br J Cancer 2006;95:1354-1361.
26. Amir E, Trinkaus M, Simmons CE, Dranitsaris G, Clemons MJ. Vascular endothelial growth factor activity after switching of bisphosphonate treatment for metastasic breast cancer. J Clin Pathol. 2009;62:474-6.
27. Lipton A. Emerging role of bisphosphonates in the clinicantitumor activity and prevention of metastasis to bone. Cancer Treat Rev. 2008;34 Suppl 1:S25-30.
28. Hashimoto K, Morishige K, Sawada K, Tahara M, Shimizu S, Ogata S et al. Alendronate suppresses tumor angiogenesis by inhibiting Rho activation of endothelial cells. Biochem Biophys Res Commun. 2007;354:478-484.














