Introduction
Soil organic matter contributes to physical, chemical and biological fertility of soil. Some practices such as reduced tillage, direct seeding, and cover crops help preserve the organic carbon content in soil. Cover crops in between rows (inter-row) of fruit plantations improve physical and chemical properties of soil (2,35). In semiarid climate, the practice also prevents fruits from becoming sunburnt (26). In the Argentina provinces of Rio Negro and Neuquén, plant species such as Festuca arundinacea, F. rubra, Trifolium pratense, T. repens, Lolium perenne and Vicia sativa are the most common cover crops used by growers (35). However, some farmers maintain their inter-row with spontaneous vegetation or bare soil.
Vegetation cover returns carbon to the soil from above-and below-ground biomass. Root-derived carbon has a longer residence time in soil than carbon derived from above-ground biomass (28). Roots contribute to a more stable carbon source (20) and increase soil carbon sequestration, which is an important aspect of sustainable farming system. Plant functional type regulates the vertical distribution of soil organic carbón (19). Root system morphology controls the availability of soil organic carbon and phosphorus (31,39) and the vertical distribution of nematodes in the soil (33). Soil fauna depends on carbon resources through the complex interactions between the roots and microorganisms (5). Microbivore nematode biomass is correlated with levels of soil carbón (41) and nematodes have also been shown to increase microbial turnover and soil N and P availability, increasing plant biomass production (16). The type of plant species present in the community, rather than the diversity of the community itself, can produce multitrophic effects on the soil food web (37). Winter cover crops, such as Hordeum vulgare and Pisum sativum, as well as Fabaceae species have been shown to increase soil food web complexity (9,21). Both the use of cover crops and the incorporation of crop straw have been shown to enhance nematodes biomass, thus facilitating carbon flow into the soil food web (41).
Previous studies on fruit orchards have focused on how the introduction of cover crops affects the nematode assemblage in different crops such as apple (30), vineyards (27), and banana (9). However, relatively little study has been conducted on soil carbon content and nematode assemblages in vegetation-covered inter-rows and vegetation-free tree rows maintained with herbicide. The present study contributes to current the understanding of how using of different long-term vegetation covers in the inter-rows of pear orchards can affect carbon flow within soil food webs. The objectives of this study were to 1) describe the relationships between nematode assemblages and the edaphic properties of the soil profile; 2) assess soil food web conditions and 3) determine the magnitude of the metabolic footprints of nematode trophic groups in a pear orchard.
Materials and Methods
Description of the study area and soil sampling
The study was conducted in a commercial pear orchard (38°51’15.7” S, 68°02’46.8” W) in the province of Río Negro, Argentina. The region has a mild continental and arid climate. The moisture regime is aridic and the temperature regime is thermic. The average temperature of the warmest month (January) is 21.9°C while the coldest month (July) averages 5.7°C. The annual average precipitation is 224 mm. Soil belongs to the Aridisol orden and is classified as Typic Acuicambid (34) with a clay loam texture at 0-20 cm (about 80% silt + clay) and silty clay loam at 20-40 cm depth.
The study was conducted in an 18-year-old pear orchard (Pyrus communis L.cv Williams) where tree spacing was 4 x 2 m (1200 pear tree ha-1). Inter-rows (i) and rows (r) in three plots were studied. The inter-row of each plot was maintained with different vegetation cover: 1) Medicago sativa L. plus grasses; (MG), 2) Festuca arundinacea; (FE), and 3) spontaneous vegetation; (SV). The pear rows were maintained without weeds with the application of herbicide. Soil samples from six treatments (MGr, SVr, FEr, MGi, SVi, FEi) and five replicates (sites) were randomly collected to assess soil properties and nematode assemblages at 0-20 and 20-40 cm depth. Each treatment was identified according to the inter-row cover of each plot. Composite soil samples of eight random sub-samples per each combination of treatment by site (n=30) and depth were collected with a soil auger (2.5 cm diameter). In each row, soil samples were taken from an area of 1 x 3 m under a selected pear tree (0.50 m and 1.50 m on both sides of a pear tree, both transversely and longitudinally to the tree rows, respectively). In each inter-row, soil samples were taken from an area similar to the row at a distance of about 1.50 m from the trunk of the selected tree from each row. Soil samples were taken in spring and autumn, from November 2012 to December 2014. The sampling dates for the FE and SV plots were as follows: the 9th of November 2012, the 30th of April and the 12th of November 2013, and the 23rd of April and the 18th of November 2014. The MG plot was sampled on the following dates: the 22nd of November 2012, the 20th of May and the 11th of December 2013, and the 28th of May and the 1st of December 2014.
Each plot was irrigated by flooding. Inter-row soil with MG (MGi) was seeded in 2004 with alfalfa (40kg ha-1) and fescue (40kg ha-1). Throughout the study, the inter-row soil was covered by 30% alfalfa, 40% Cynodon dactylon and 30% F. arundinacea plus Plantago lanceolata. Fescue inter-row (FEi) was seeded in 2005. At the time of the study, the vegetation cover of the FEi was fescue and 10% Cynodon dactylon. Plant species composition in the SV inter-row (SVi) was 45% C. dactylon and 21% Trifolium repens. Of the remaining plants (34%), a mix of Taraxacum officinale, Trifolium pratense, Cichorium intybus, P. lanceolata, Lactuca serriola, Polygonum aviculare and Sonchus oleraceus were recorded. The inter-row was seeded to a width of 3 m starting at 0.50 m from the trunk of each pear tree. Vegetation cover was mowed three or four times during summer (2012-13, 2013-14), and the clippings were left on the soil surface to decompose. Part of the above-ground plant biomass was collected in a 1 x 1 m quadrant in the inter-row space of each treatment. Shoots and leaves were dried in an oven at 60°C to a constant weight to determine dry matter production. Tree rows were managed according to the traditional practices employed by growers in this region. In each growing season, trees were fertilized in spring at 100 units of nitrogen per hectare, half in October-November and half in December. In autumn, fertilization was completed with 15 units of nitrogen per hectare. Fertilizers were applied in the tree rows, including ammonium sulfate, solMIX, phosphoric acid and diammonium phosphate. Herbicides were used to achieve weed control. For each growing season, 1,1’-dimethyl-4-4’-bipyridinium dichloride was applied 2-3 times at a rate of 3.5 L ha-1 in spring and glyphosate was applied 4-5 times in summer at a rate of 4 L ha-1.
Soil physicochemical and nematode analyses
Soil organic carbon (SOC) was determined by wet oxidation (K2Cr2O7). Soil salinity was measured from the extracted saturation and expressed as electrical conductivity (EC). Sodicity was expressed as exchangeable sodium percentage (ESP). Exchangeable potassium (K) and sodium were measured with a flame spectrometer after exchange with ammonium acetate. Available phosphorus (P) in soil was determined by the Olsen method. Total nitrogen was measured using the Kjeldahl method only at the first time of sampling at 0-20 cm depth.
Soil nematodes were extracted from 100 g of fresh soil using centrifugal-flotation (6). Soil moisture was determined gravimetrically by drying the samples at 105°C for 24 h. Nematode counts for each taxon were adjusted to the number of nematodes per 100 g of dry soil. The recovered nematodes were counted and preserved in formalin. At least 100 specimens from each sample were randomly selected and identified to genus or family level to be assigned to five trophic groups: bacterivores, fungivores, obligate plant feeders, facultative plant feeders, and omnivores-predators according to Yeates et al. (1993). The abundance of nematode trophic groups and the trophic diversity index (T = 1/Σpi 2, pi is the proportion of the trophic group i in nematode assemblage) were calculated to describe the nematode assemblage. The enrichment index (EI), structure index (SI) and, channel index (CI) were calculated to assess the condition of the soil food web according to Ferris et al. (2001). Nematode biomass and metabolic footprints were calculated using NINJA´s on-line web application (32). Metabolic footprint informs on the ecological function undertaken by nematodes (14).
Statistical analyses
Analysis of variance of a linear mixed model was employed to compare the fixed effects of treatment and season on soil physicochemical data, nematode abundance, ecological indices, and metabolic footprints using the statistical software Infostat (2020). Each soil depth was investigated separately. A random effect was included to model the variability of sites within the plots. Differences obtained at the p < 0.05 level were considered significant using the LSD Fisher test. An analysis of variance was performed to test for differences in dry matter production per cover. Principal component analysis (PCA) was performed to explore the relationship between nematode trophic groups and soil physicochemical properties under different treatments and depths. A Pearson’s correlation analysis was performed to quantify the association between SOC and biomass of nematode assemblage in the row and the inter-row in the 0-40 cm soil profile using Infostat (2020).
Results
Soil physicochemical properties and plant dry matter production
At 0-20 cm depth, SOC was higher in MGi in spring than in other treatment x season interactions (Table 1, page 89). In spring and autumn, SOC was higher in MGi than in MGr. The value of total N in MGi was 0.15% and higher than the other treatments (p<0.01). Available P was higher in the rows and SVi than in FEi and MGi. Exchangeable K was higher in MGi in spring than in other treatment x season interactions. The highest values of EC and ESP were obtained in FEi, and were greater in autumn than in spring. At 20-40 cm depth, SOC was greater in MGi than under all other treatments which did not vary from each other. The average EC value was higher in FEi (Table 1, page 89).
Table 1 Main values of soil properties (±SE) in six treatments at two depths. Tabla 1. Valores medios de las propiedades del suelo (±ES) en seis tratamientos a dos profundidades.
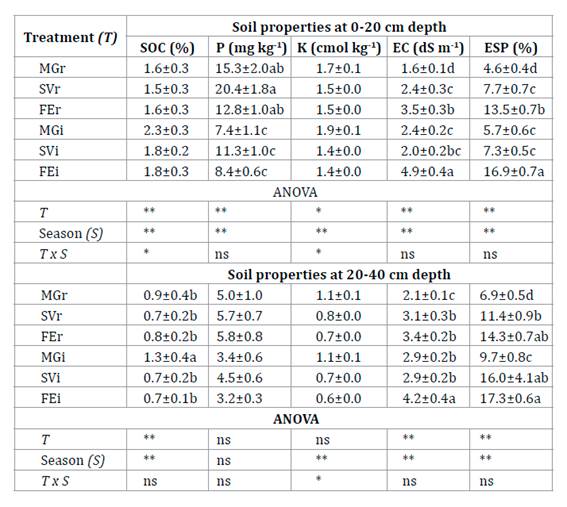
Each treatment was identified according to the inter-row cover of each plot: Medicago+grasses; MG, spontaneous vegetation; SV and fescue; FE. r: row and i: inter-row. SOC: Soil organic carbon, K: Exchangeable potassium, P: Available phosphorus, EC: Electrical conductivity and ESP: exchangeable sodium percentage. Different letters in the column indicate significant differences between treatments for analysis of variance at * P<0.05, ** P<0.01, and ns: not significant according to Fisher test.
Cada tratamiento fue identificado de acuerdo con la cobertura del interfilar de cada parcela Medicago+grasses; MG, vegetación espontánea; SV y festuca FE. r: fila e i: interfilar. SOC: Carbono orgánico del suelo, K: Potasio intercambiable, P: Fósforo disponible, EC: conductividad eléctrica, y ESP: porcentaje de sodio intercambiable. Diferentes letras en la columna indican diferencias significativas entre tratamientos para el análisis de varianza a * P<0,05, ** P<0,01, y ns: no significativo según la prueba de Fisher.
Plant dry matter production was higher in the MGi than in other soil covers (p<0.01). The amount of dry biomass was 14.3, 8.1 y 6.5 t ha-1 for the MG, FE and SV inter-rows, respectively.
Nematode abundance
Total nematode abundance was higher in each inter-row than in its associated row (Table 2, page 90). At 0-20 cm, bacterivores were most abundant in the rows, whereas different trophic groups predominated in abundance in the inter-rows. The 20-40 cm layer was generally dominated by obligate plant feeders, followed by bacterivores.
Table 2 Mean abundance of nematode (individuals 100g-1 dry soil ±SE) in six treatments at two depths. Tabla 2. Abundancia media de nematodos (individuos en 100g-1 de suelo seco ±ES) en seis tratamientos a dos profundidades.
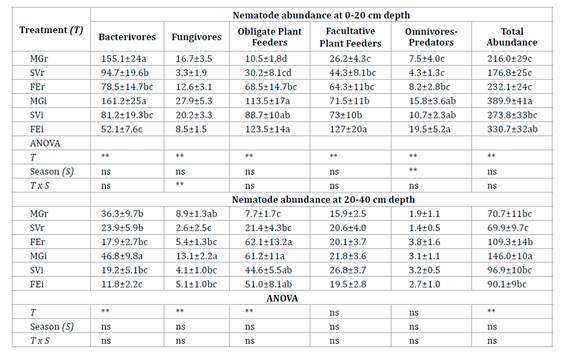
Each treatment was identified according to the inter-row cover of each plot: Medicago+grasses; MG, spontaneous vegetation; SV and fescue; FE. r: row and i: inter-row. Different letters in the column indicate significant differences between treatments. Significance levels: *P<0.05, **P<0.01, and ns: not significant according to Fisher test.
Cada tratamiento fue identificado de acuerdo con la cobertura del interfilar de cada parcela Medicago+grasses; MG, vegetación espontánea; SV y festuca FE. r: fila e i: interfilar. Diferentes letras en la columna indican diferencias significativas entre tratamientos. Niveles de significancia: *P<0,05, **P<0,01, y ns: no significativo según la prueba de Fisher.
At 0-20 cm depth the abundance of bacterivores was higher in MGi and MGr than in other treatments (Table 2, page 90). The abundance of fungivores was higher in MGi and SVi. The population density of obligate plant feeders did not vary between MG, SV and FE inter-rows. The highest density of facultative plant feeders was observed in FEi and was higher in each inter-row than its associated row. The population density of omnivore-predators was greater in the inter-rows than in the rows, and greater in spring than in autumn. The abundance of bacterivores at 20-40 cm depth showed a similar trend as at 0-20 cm depth (Table 2, page 90). The abundance of fungivores was higher in MGi and MGr. Obligate plant feeders were more abundant in the inter-rows and FEr.
Linking the nematode assemblage with soil physicochemical properties
An exploratory PCA showed the relationships between the nematode assemblage and soil properties in different treatments and soil profiles (Figure 1, page 90).
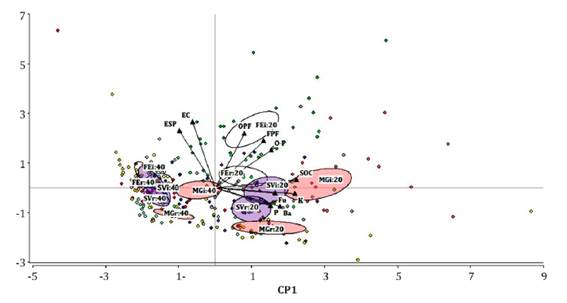
Confidence ellipses around each treatment are shown: MGr20 and MGi20: row and inter-row of plot with Medicago+grasses cover at 0-20 cm. SVr20 and SVi20: row and inter-row of plot with spontaneous vegetation cover at 0-20 cm. FEr and FEi: row and inter-row of plot with fescue cover at 0-20 cm. The same references are repeated to 20-40 cm depth. SOC: Soil organic carbon, K: Exchangeable potassium, P: Available phosphorus, EC: Electrical conductivity, ESP: exchangeable sodium percentage, Ba: Bacterivores, Fu: Fungivores, OPF: Obligate plant feeders, FPF: Facultative plant feeders, and O-P: Omnivores-Predators.
Las elipses de confianza se observan por cada tratamiento: MGr and MGi: fila e interfilar de la parcela con la cobertura Medicago+grasses a los 0-20 cm. SVr and SVi: fila e interfilar de la parcela con vegetación espontánea a los 0-20 cm. FEr and FEi: fila e interfilar de la parcela con la cobertura festuca a los 0-20 cm. Las mismas referencias se repiten para 0-40 cm. SOC: Carbono orgánico del suelo, K: Potasio intercambiable, P: Fósforo disponible, EC: Conductividad eléctrica, ESP: Porcentaje de sodio intercambiable, Ba: Bacteriófagos, Fu: Fungívoros, OPF: Fitófagos obligados, FPF: Fitófagos facultativos y O-P: Omnívoros-Predatores.
Figure 1 Biplot of principal component analysis based on the soil properties and nematode trophic groups at two depths. Figura 1. Gráfico del análisis de componentes principales sobre las propiedades del suelo y los grupos tróficos de nematodos a dos profundidades
The first two principal components, PC1 (36.4%) and PC2 (17.1%), explained 53.5% of the variance. PC1 was strongly and positively correlated with SOC, K, and the abundance of bacterivores and fungivores. PC2 was positively correlated with the abundance of obligate plant feeders, EC and ESP. A grouping pattern according to soil depth can be observed along the PC1 axis. The nematode assemblage was generally different among treatments at 0-20 cm depth. In contrast, at 20-40 cm, a clustering pattern between FE and SV inter-rows and rows was observed.
Nematode community indices, biomass and metabolic footprints of soil nematodes
At 0-20 cm depth, EI was higher in MGr and MGi (Table 3). In general, SI was higher in each inter-row compared to its row. CI was higher in SVi than in the other inter-rows. At 20-40 cm depth the values of EI, SI, and CI did not vary between treatment and season (Table 3).
Table 3 Mean values of nematode indices (±SE) in six treatments at two depths. Tabla 3. Valores promedio de los índices de nematodes (±ES) en seis tratamientos a dos profundidades.
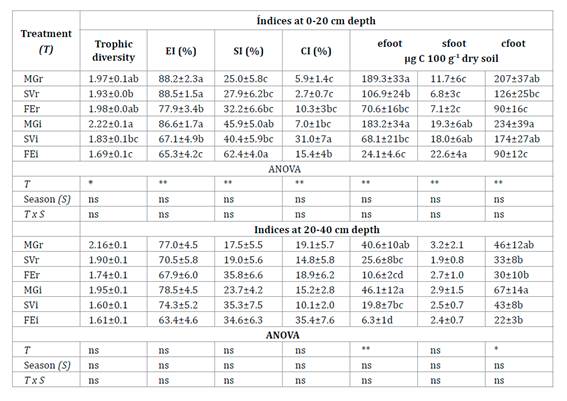
Each treatment was identified according to the inter-row cover of each plot: Medicago+grasses; MG, spontaneous vegetation; SV and fescue; FE. r: row and i: inter-row. EI: enrichment index, SI: Structure index. CI: Channel index, efoot: enrichment footprint, sfoot: structure footprint, and cfoot: composite footprint. Different letters in columns indicate significant differences between treatments. Significance levels: *P<0.05, **P<0.01, and ns: not significant according to Fisher test.
Cada tratamiento fue identificado de acuerdo con la cobertura del interfilar de cada parcela Medicago+grasses; MG, vegetación espontánea; SV y festuca FE. r: fila e i: interfilar. EI: Índice de enriquecimiento, SI: Índice de estructura, CI: Índice canal, efoot: huella de enriquecimiento, sfoot: huella de estructura, y cfoot: huella compuesta. Diferentes letras en las columnas indican diferencias significativas entre tratamientos. Niveles de significancia: *P<0,05, **P<0,01, y ns: no significativo según la prueba de Fisher.
In the 0-40 cm soil profile, the biomass of nematode assemblage showed a positive correlation with the content of SOC in the row (r=0.48, p<0.01) and in the inter-row (r=0.46, p<0.01). At 0-20 cm depth, nematode composite footprint was higher in MGi and SVi than FEi (Table 3). Bacterivores accounted for the highest metabolic footprint in the rows, while bacterivores and herbivores were prominent in the inter-rows (Figure 2a, page 92).
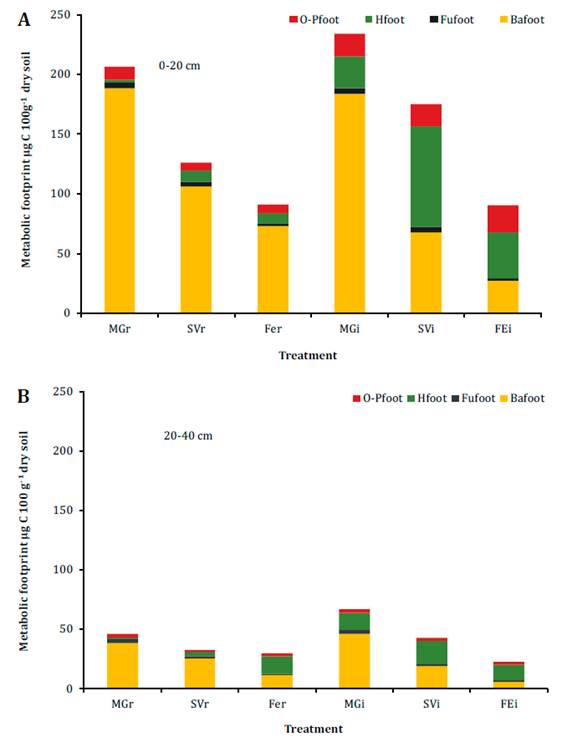
Each treatment was identified according to the inter-row cover of each plot: Medicago+grasses; MG, spontaneous vegetation; SV and fescue; FE. r: row and i: inter-row. Ba: Bacterivores, Fu: Fungivores, H: Herbivores, and O-P: Omnivores- Predators.
Cada tratamiento fue identificado de acuerdo con la cobertura del interfilar de cada parcela: Medicago+grasses; MG, vegetación espontánea; SV y festuca FE. r: fila e i: interfilar. Ba: Bacteriófagos, Fu: Fungivoros, H: Herbívoros, y O-P: Omnívorospredatores.
Figure 2 Metabolic footprint (foot) of nematode trophic groups in six treatments at (A) 0-20 and (B) 20-40 cm depths. Figura 2. Huella metabólica (foot) de los grupos tróficos de nematodos en seis tratamientos a las profundidades (A) 0-20 y (B) 20-40 cm.
Bacterivore footprint was higher in MGi and MGr than in the other treatments (p<0.01). Herbivore footprint was highest in SVi (p<0.01, Figure 2a, page 92) and was greater in the inter-rows than in the rows (p<0.01). MGi and MGr were associated with a higher enrichment footprint (Table 3). Structure footprint was higher in the inter-row tan in its associated row. At 20-40 cm depth, composite footprint was higher in MGi than in the other inter-rows, while it was similar among rows (Table 3). Herbivore footprint did not vary among inter-rows (p<0.03, Figure 2b, page 92). MGi and MGr were associated with a higher enrichment footprint (Table 3).
Discussion
Soil physicochemical properties and nematode assemblages at different soil depths
Vegetation cover significantly impacted soil organic carbon levels in the studied pear orchard. Medicago+grasses-covered inter-rows exhibited SOC levels that were 22% and 46% higher at depths of 0-20 and 20-40 cm, respectively, than other covers at those depths. SOC provides resources for soil biota (31,41) and this may be reflected by the positive association seen between soil carbon and nematode biomass in the soil profile. Stratification of SOC is common in agroecosystems (17). In MGi, the stratification of SOC was less noticeable than in the SV and FE inter-rows. Plant functional types and architectural root characteristics regulate the vertical distribution of soil organic matter (19). Vegetal species such as Medicago sativa, which has a vigorous root system in deep soil, contribute to carbon storage in the soil profile because the carbon produced by roots forms stable organo-mineral associations (20). In the present study, maintaining a permanent cover with MGi in springtime resulted in a higher level of SOC in the soil profile compared to MGr. Interestingly, this increase in organic matter -evidently brought about by MG cover- coincided with the period when new pear roots are developed, thus ensuring that nutrients are available for fruit crop uptake.
Total nematode abundance showed a differential distribution between the soil layers; about 75% of all nematodes recorded at 0-40 cm depth were recovered from the top 0-20 cm. This differential distribution pattern applies to both inter-rows and rows alike. Previous studies have shown a similar trend (31,39). Total nematode abundance was generally greater in inter-rows than in rows at a depth of 0-20 cm (Table 2, page 90). In the inter-row of each plot, populations of fungivores, facultative and obligate plant feeders, and omnivores-predators were seen in greater abundance than in the row. Using herbicides to control weeds in the pear row could alter the nematode assemblage. Seedling emergence and root length were reduced by application glyphosate on pre-existing vegetation (29). The loss of plants due to weeding may result in a loss of food resources, thereby affecting several trophic groups (25).
Environmental and edaphic factors shape spatial heterogeneity of soil according to land use and root distribution (24). Nematode assemblages will therefore differ depending on resource availability and soil spatial heterogeneity along the depth gradient (13). In the topsoil, each inter-row recorded different nematode assemblages that were associated with particular edaphic properties. In MGi, bacterivore nematodes dominated in a non-saline soil with the highest content of SOC, total N and exchangeable K, which is in line with other studies in Fabaceae crops (9,37). Bacterivores are attracted by the volatile compounds released by legume roots, which lead to the spread of bacteria and improve rhizobial inoculation (18). In the present study, total soil N supply was 30% higher under Medicago+grasses cover tan any other cover. Nearby trees can capture this inter-row nitrogen. The pear tree develops roots with a greater exploratory capacity when grafted on rootstock, and these roots exceed the canopy´s projection on the ground, this allows the plant to capture the available nitrogen in the inter-row. In SVi, obligate plant feeders and bacterivores were similar in abundance and were the most abundant groups. The highest plant species richness and available P content in non-saline soil were associated with nematode assemblages´ trophic structure. Floristic diversity has a potential role in soil P input due to a differential accumulation of P in each plant (23), which contributes to an increasing this nutrient of limited mobility in the soil profile. In the present study, P was probably released by the decomposition of plant residues from vegetation that was mown over the summer. Furthermore, frosts in Winter and spring are common in the study region and cause the death of shoot and root cells, releasing intracellular P (22). In FEi, obligate and facultative plant feeders were found to be the most abundant groups in slightly saline and sodic soil. The index of nematode trophic diversity recorded its lowest value in this treatment compared to other treatments. This finding concurs with Su et al. (2016), who found a sensitivity of this nematode trophic group to slightly saline soils. For a detailed view of the composition of soil nematode plant feeders see Azpilicueta et al. (2017).
In the sub-superficial soil, in the inter-rows of SV and FE, obligate plant feeders dominated, while in the pear rows, plant feeders and bacterivores prevailed in saline and sodic soil. A previous study on a pear orchard observed that plant feeders were the most abundant trophic group in saline and slightly sodic soil (3). In the valleys of Río Negro and Neuquén, about 40% of soils from fruit orchards have a shallow water table, which impacts the salinization process of soil (15) because the water table fluctuates to different depths during the crop growing season (1).
Soil food web condition
In the topsoil, the higher enrichment index (EI) found in the MGi suggests a more enriched condition of the soil food web than in the SV and FE inter-rows. A positive relationship between EI and quantity of cover crop biomass was found by Dupont et al. (2009). In the present study, plant dry matter production was ca. two times higher in the MGi than in the other inter-rows. The return of plant residues to the soil contributes to the availability of basal resources for primary decomposers in the soil food web (38). This was reflected in the fact that the highest bacterivore and fungivore abundance observed in our study was recorded in MGi. These trophic feeding groups have shown to be first responders to resource enrichment (41).
Microbivore nematodes are at the base of the soil food web and drive energy flow to the higher trophic groups into the bacterial or fungal energy channel. The channel index values were less than 30% in the inter-rows and rows, indicating that the bacterial channel was dominant compared to the fungal channel (12). The magnitude of soil energy channels can be estimated using both biomass and metabolic footprint attributes (7,14). The bacterivore metabolic footprint was higher in MGi than in SVi and FEi in the soil profile. Furthermore, the bacterivore footprint represented 70% of the composite footprint, indicating that the highest carbon assimilation in the soil food web occurred through bacterial channels. Another soil energy channel is mediated by plant feeding and involves live roots. Herbivore footprints were higher in the inter-rows than in the rows, particularly in SVi, which could reflect the heterogeneity of resources due to greater vegetation diversity. Plant species identity has relevance in controlling energy channels in soil systems (7).
Structure index and footprint were low to moderate across all treatments; consequently, the magnitude of pest suppression performed by omnivores-predators was low. Due to the high content of fine particles, the movement and reproduction of omnivores-predators could be affected by granulometric soil composition. In addition, the predominance of small pores could reduce the abundance of the largest nematodes in the soil profile (33). Even so, SI and sfoot were higher in the inter-rows than in the rows, suggesting a more complex nematode assemblage with more linkages in the food web in the inter-rows. The inter-rows with vegetation covers were reservoirs of omnivores-predators, which can regulate lower soil food web levels, including plant-feeders. The structure footprint was lower than the enrichment footprint in both rows and inter-rows. According to Ewald et al. (2020), food webs in arable systems have low productivity and high resource input. The mineralization service given by the enrichment footprint was higher in MGi and MGr. The excretory products of nematodes, such as amino acids, NH4 + and PO4 -3 increase soil nutrient availability (16), and they may improve the growth and production of fruit and cover crops.
Conclusions
This study has enhanced the current knowledge of carbon flow in the soil food web in the inter-rows and rows of a pear orchard. Bacterial channels prevailed in the rows, while different energy channels were found in the inter-rows. The relative magnitude of soil energy channels revealed higher carbon flow within the soil food web under Medicago+grasses and spontaneous vegetation than under fescue. The herbivore energy channel made a significant contribution under spontaneous vegetation.
Our data suggest that nutrient mineralization was greater in inter-rows and rows in the Medicago+grasses plot. Moreover, a higher soil carbon content was seen in the inter-row under Medicago+grasses, and this treatment also yielded a higher abundance of bacterivores and fungivores in the soil profile, and was thus seen to have an impact on soil fertility.
Each cover crop was associated with a different nematode assemblage due to their particular edaphic properties, mainly in the topsoil. Nematode biomass was positively correlated with soil carbon content. The abundance of omnivores-predators associated with the structure of soil food web was greater in the inter-rows than in the rows. Consequently, pear rows with disturbed soil food webs could be improved with the assistance of nematodes from associated inter-rows. Taken together, these findings suggest that adequate soil management in inter-rows may promote the abundance of beneficial nematodes and improve the productive capacity of pear trees.