Introduction
Nowadays, breeding dogs requires a competent, science-based approach. High breeding value, the uniqueness of some individuals is a prerequisite for a thorough examination of the reproductive system of bitches not only to exclude infectious diseases, but also to identify the timing of ovulation to use them in reproduction (Morrow, 1986; Freshman, 1991; Johnston et al., 1994; Goodman, 2001; Arlt, 2018; Stekol'nikov et al., 2018). Determination of ovulation is especially relevant for animals that are intended for mating in other regions of the country and the world (England & Concannon, 2002; Derkach, 2011; Plemjashov et al., 2016).
One of the important factors of successful mating or artificial insemination of bitches is choice of the optimal insemination time (Hase et al., 2000; De Gier et al., 2006; Allen, 2002; Miroljubov et al., 2003; Lévy & Fontbonne, 2007; Sorokoletova & Shmidt, 2008; Pardo-Carmona et al., 2011; Plemjashov et al., 2016; Djul'ger & Djul'ger, 2018). Different bitches have a long period of heat and high variation of its duration, which requires an individual approach to the choice of insemination time. Usually, by the time of the planned mating, the owners of bitches have observed the heat several times, know its duration, intensity, and can predict the date of mating with some accuracy. However, subjective observations are often not enough for successful artificial insemination, especially when it comes to the use of valuable sperm, as well as if its amount is limited (Nizanski et al., 2004; Derkach, 2011; Pardo-Carmona et al., 2011; Djul'ger & Djul'ger, 2018; Dobrjanskaja & Harina, 2019).
Determining the optimal service time (both natural and artificial) is one of the factors of infertility, because it determines fertility (Hewitt & England, 2000; Allen, 2002; England & Concannon, 2002; Davidson, 2007; Pardo-Carmona et al., 2011 ; Karnouhova, 2011a, 2011b; Labib et al., 2018). The sexual cycle in bitches is physiologically manifested by significant variations that can be perceived as pathology. There are cases when, during physiological estrus, bitches do not become pregnant (fertilization), which can be a significant problem. In fact, more than 40% of problems related to the fertility of breeding bitches are due to inaccuracy in determining the time of insemination (Zoldag et al., 1993; Derkach, 2011; Karnouhova, 2011a).
It is known that the optimal days for mating bitches differ in dogs of one breed (Phemister et al., 1973; Hori et al., 2012; Hahn et al., 2017) and may even "shift" during life, even in the same animal (Grishina & Minjuk, 2016). It is believed that with increasing age of the bitch it should be mated at a later date. This is usually true, but there are many cases where, on the contrary, estrus, which usually coincides with the ovulation period, can occur earlier than in previous heats. In addition, there are "instances" in which sexual intercourse does not coincide with the period of ovulation - it can occur later on the days when most likely to get pregnant (Hewitt & England, 2000; Goodman, 2001; Derkach, 2011).
At the same time many breeders carry out mating of the bitches in the standard mode, in a certain number of days after the blood vaginal secretions noticed for the first time (Allen, 2002; Von Heimendahl & England, 2010; Karnouhova, 2011b; Bordjugov et al., 2013). This is often the most common cause of apparent infertility because mating occurs at the wrong time. Most owners practice mating (insemination) for 10-14 days from the beginning of proestrus, but this does not always give the expected result, as ovulation can occur on 21 and even 27-31 days (England & Concannon, 2002; Niżanski W. 2004; Kustric & Dzhonston, 2005; Bicudo et al., 2010; Derkach, 2011; Hori et al., 2012; Davidenko & Ponomarenko, 2016; Djul'ger & Djul'ger, 2018).
Taking into account the duration of gametes ability to fertilize is one of the factors of effective insemination: the lifespan of sperm in the female genitalia is limited to 2-3 days, while the оocyte after ovulation retains its viability for one day, and fertilization for 10-16 hours. Therefore, fertility, both for too early and for late insemination, will be reduced, causing infertility (Tsutsui, 1988; Allen, 2002; Berezovs'kyj & Harenko, 2017).
It is known that the optimal time for insemination is close to the time of ovulation, which usually occurs 1-3 days from the start of the hunt, less often 2 days before its onset or delayed up to 5-7 days of estrus. Mature follicles ovulate for 12-24 hours. That is, the optimal time of insemination is 2-4 days from the manifestation of estrus (England & Concannon, 2002; Simpson et al., 2005; Thomas, 2013; Berezovs'kyj & Harenko, 2017).
Therefore, there is a problem of choosing the optimal method of determining the time of ovulation for the selection day of insemination (Goodman, 2001; Derkach, 2011; Grishina & Minjuk, 2016; Hahn et al., 2017; Labib et al., 2018; Plemjashov & Plahova, 2018). In addition, it has an economic aspect, because owners often have to carry bitches for mating for a long distance and they must be sure of the exact date of ovulation (Pardo-Carmona et al., 2011; Bordjugov et al., 2013).
Methods for determining the optimal insemination time of bitches
To determine the fertile period and the optimal timing of insemination, various methods are used, which include clinical, vaginal cytology, vaginal endoscopy, analysis of the concentration of hormones in the serum and others (Jeffcoat & Lindsay, 1989; Freshman, 1991; England & Concannon, 2002; Fáy et al., 2003; Uillard et al., 2004; Simpson et al., 2005; Pardo-Carmona et al., 2011; Moxon et al., 2012; Bordjugov et al., 2013; Radohlib & Krajevs'kyj, 2014; Togoe et al., 2014; Hahn et al., 2017; Labib et al., 2018; Plemjashov & Plahova, 2018).
Clinical (clinical-visual) method
This is one of the simplest and most affordable methods of determining the optimal insemination time of bitches. Based on the detection clinical signs in animals and behavioral changes characteristic of the stages of the sexual cycle with the peak of estrus (estrus behavior – acceptance of male): a positive sexual reaction of the female to the male (Concannon, 2011; Hahn et al., 2017).
Estrus in dogs lasts an average of 9 (2 ... 16) days. Discharge from the genital slit is usually light or with traces of blood. Vulva become less swollen and more rigid. The female shows an increased "interest" in males, sniffs their genitals; then chooses a male favorite, allows mount and coitus (Bordjugov et al., 2013).
So, some breeders often use a simple method of counting days, determining the time of ovulation after a certain number of days that have passed since the onset of proestrus (the first bloody discharge). Indeed, the average bitch ovulates 10-12 days after the onset of proestrus, but this situation may develop in another scenario: in some bitches ovulation occurs approximately 5 days after the onset of proestrus, in others - in 30 days, and therefore mating on 11-13 days, which is widely practiced by breeders, may not lead to a positive result, and the bitch will remain "empty" (England & Concannon, 2002; Thomas, 2013).
Assessment of the vulva. During the proestrus phase, the vulva increases in size and becomes swollen. In the estrus phase, it decreases slightly and becomes softer. Softening of the vulva occurs when a special hormone is released into the bloodstream, which coincides with the onset of estrus and stimulates the bitches to behave accordingly - the bitch is ready to allow coitus. However, many bitches do not allow dogs, even when ready to mate, and their behavior cannot be guided. If only a clinical assessment of the timing of mating is available, the breeder in his decision should proceed from the beginning of estrus and softening of the vulva, because both of these phenomena occur, on average, 4 days before the start of the fertilization period (Allen, 2002; England & Concannon, 2002; Thomas, 2013; Hahn et al., 2017). Therefore, it is best to carry out mating 4 days after the set day of estrus.
Evaluation of vaginal discharge. In the estrus phase, vaginal discharge becomes less abundant, lighter, possibly mucous. In appearance resemble meat juice. But some bitches retain bloody discharge, and their intensity does not change compared to proestrus (Thomas, 2013).
And even the diagnosis of signs of estrus (positive reaction of the female to the male) are only indirect signs of ovulation, because some females allow mating from the beginning of proestrus, and ovulation occurs much later - even at 30 days. Many bitches mate during a false pregnancy, infectious inflammation of the urinary tract, the presence of ovarian cysts, the manifestation of nymphomania. That is, the behavior of many bitches may not coincide with the hormonal background that promotes fertilization (Allen, 2002; England & Concannon, 2002; Lévy & Fontbonne, 2007; Bordjugov et al., 2013; Thomas, 2013; Davidenko & Ponomarenko, 2016).
Thus, clinical and visual examination of bitches does not always guarantee the onset of ovulation and can be used only in combination with some other methods to determine the optimal time of insemination.
Evaluation of saliva crystallization
At the heart of all methods of determining the time of ovulation by saliva is the ability to detect in this biological fluid salts that crystallize as a result of increasing the amount of estrogen in the body (England, 1992; England & Concannon, 2002; Soljannikova & Brjuhin, 2017). You can see them through the use of specially designed devices. In the initial period of the cycle, the picture will look like dotted lines, and a little later the transverse lines join.
Crystallization of mucus from the vagina and cervix of women with the formation of tree-like structures was first described by Papanicolau in 1942 (Papanicolau, 1942). This method is that by analyzing under a microscope the change in the nature of the crystallization of dried saliva (or mucus) can be judged with a high degree of probability of the onset of the fetal or infertile period and, accordingly, increase the likelihood of pregnancy (Pardo-Carmona et al., 2011). Garm and Skjerven (1952) studied the crystallization of mucus from the vagina and cervix of cows; they noted that the crystals in the form of branches were formed during heat and disappeared in the luteal phase of the cycle. In women, cyclic changes in the nature of crystallization are associated with the secretion of estrogen and progesterone; estrogen promotes crystallization, while progesterone inhibits it. Zondek and Cooper noted that not only cervical mucus crystallizes, but also all mucous secretions and most body fluids, including saliva (Zondek & Cooper, 1954). It turned out that the crystals are a common salt, and their shape depends on the presence of mucin. Probably, the crystallization process also depends on the presence of electrolytes, protein and carbohydrates (Pardo-Carmona et al., 2011).
Thus, as estrogen increases in the body and increases the number of crystals in saliva, and on the day of ovulation, the crystals acquire a shape resembling a fern. This method in medicine is called "arborization", from the Latin "arbor" - a tree (Raeside & McDonald, 1959; England, 1992; England & Allen, 1989; Voroncova, 2008; Masalovych et al., 2018).
The sensitivity and specificity of the test with saliva crystallization is indicated as 53 and 72% (Braat et al., 1998). Based on these features, ready-made test systems have been developed that allow you to quickly, easily and inexpensively determine the period favorable for fertilization.
With the advent of test minimicroscopes the arborization method became available at home.
According to some scientists, the method is quite subjective and physiologically imperfect, as indicated by the possibility of crystallization of mucus during anovulatory sexual cycles and ovarian cysts (Shabanah, 1960; Bordjugov et al., 2013; Thomas, 2013; Plemjashov & Plahova, 2018). It is possible that the combination of this test with others can be a more accurate means of diagnosing the optimal insemination time in bitches, although further research is needed to confirm this. (Perez et al., 2005; England & Allen, 1989; Pardo-Carmona et al., 2011).
Electrometric method of examination of cervico-vaginal secretion
Based on the fact that the electrical resistance of the vaginal mucosa decreases in the late phase of estrus (Ramos et al., 2001; Allen, 2002; England & Concannon, 2002; Hahn et al., 2017). Unlike females of other animal species (cattle, sheep, and pigs), the electrical resistance of vaginal secretions in bitches increases during proestrus and remains high, while bitches exhibit estrogen behavior and a high percentage of eosinophilic surface cells in the vaginal swab.
The relationship between the electrical resistance of the vaginal mucosa and the concentrations of estradiol and progesterone in the serum during the estrous cycle was studied. It was found that the impedometric characteristics of vaginal mucus are an indicator of serum concentrations of progesterone and its correlation with estrogen during the terminal stage of the estrous cycle in cyclic females (Bartlewski et al., 1999).
Ovulation, which is determined by an increase in serum progesterone levels above 5 ng / ml, occurs during a period of increased resistance. Optimal conditions for conception with the last 3 days of increased resistance (Günzel et al., 1986).
The correlation between the electrical resistance of the vaginal membrane and the preovulatory concentration of luteinizing hormone is proved. As the follicles mature, the electrical resistance (450-600 ohms) decreases sharply. During heat, when the resistance of the vaginal mucosa is 200-300 ohms, the cervix is always open, and when the resistance is more than 350 ohms, it can be closed or slightly open. The optimal time for insemination is marked at 250-400 ohms (Leidl & Stolla,1976; Bordjugov et al., 2013).
Gűrler et al. note that vaginal electrical impedance was a faster and cheaper method than progesterone assessment and more reliable than vaginal cytology and clinical assessment. In conclusion, the authors note that the combination of vaginal measurements of electrical impedance and progesterone assessment was a more useful method for determining the optimal insemination time in bitches (Gűrler et al., 2018).
The use of estrous detectors (heat detectors, estrometers) for this purpose allows to determine the onset of the fertile period at home (Plemjashov & Plahova, 2018). In particular, in the Rocha Fonseca study, tests with the Draminski ovulation detector showed that 87.5% of bitches were close to ovulation. The natural alternating current of the genitals is amplified at the beginning of heat, biologically active points seem to experience a conditional imbalance, thereby increasing bioelectrical activity, which reduces the conductivity. The electrical conductivity increases sharply at the relevant biologically active points, which are responsible for the function of the ovaries and uterus associated with ovulation. They conclude that using this test you can determine the optimal time for natural or artificial insemination, avoiding unnecessary costs for breeders (Rocha Fonseca, 2016).
Most authors point to the insufficient efficiency of estrual detectors, but at the same time note that its use with other methods significantly increases the effectiveness of measures to determine the optimal insemination time of bitches (Von Heimendahl & England, 2010; Jang et al., 2013; Antonov et al., 2014).
Ljubec'kyj et al. (2010) studied the correlation between changes in the electrical conductivity of biologically active points of the uterus and ovaries during heat with the optimal insemination time of bitches. It was found that the dynamics of the electrical conductivity in the biologically active points of the skin, which are responsible for the function of the uterus and ovaries, is associated with morpho-functional changes in the genitals of bitches during heat and reflects their bioelectrical activity. They conclude that the use of this method for the diagnosis of the optimal insemination time of bitches is quite useful, but due to the complexity of application and especially the interpretation of the results, its wide implementation in practice is limited (England & Concannon, 2002; Thomas, 2013). However, the deep scientific value of electropuncture diagnostics to determine the optimal time of insemination of bitches is not lost.
In general, it should be noted that the methods of cervical-vaginal secretion are still poorly understood in bitches (Allen, 2002).
Vaginal endoscopy (vaginoscopy)
Cessation of estradiol secretion by follicles and their transition to progesterone secretion leads to a decrease in both edema and vascularization of mucous membranes, accompanied by a pronounced change in the nature of vaginal discharge, which in turn is detected by vaginal endoscopy. At the stage of proestrus, the mucous membrane becomes more convex and swollen, then there is a decrease; as the ovulatory peak approaches, the mucosa shrinks and pales, in some cases becoming almost white. These changes indicate the approach of the stage, which is characterized by a gradual increase in the concentration of progesterone before ovulation and the subsequent onset of the fertile period. These changes are easy to recognize, even with a little practice, in addition, they are so reproducible that a set of features they can be evaluated semi-quantitatively.
A simple and convenient method that provides fairly reliable information to determine the optimal mating time. This method is to determine the condition of the vaginal mucosa, namely its folds and color (Lindsay & Concannon, 1986; Thomas, 2013; Plemjashov & Plahova, 2018). During proestrus, the color of the vagina is pale, the folds have a dull cream color. In the beginning phase of estrus, the folds of the mucosa are swollen, pink, increased in size. The period of fertility is determined by the reduction of the mucosa and the formation of lumpy folds. Mucosal contraction begins 3-1 days before ovulation, so mating should be carried out within 3-5 days after the first observed contraction of the mucosa. The period of fertilization ends with the change or cessation of contraction of the mucous membrane, which gradually turns pink (Allen, 2002; England & Concannon, 2002).
Vaginal endoscopy is very useful in determining the optimal timing of mating. At the end of the fertile period, ie at the beginning of metestrus, the vaginal mucosa pales and thins, the folds become rounded - and probably the most characteristic feature - the mucosa in the front of the vagina looks irritated and quickly compresses when touched, forming a rosette (Lindsay, 1983; Simpson et al., 2005).
The significant cost of equipment and manipulations on it, as well as certain inconveniences that arise during the study of aggressive and restless bitches, are the disadvantages of this method (England & Concannon, 2002; Derkach, 2011; Bordjugov et al., 2013; Plemjashov & Plahova, 2018). However, some authors see the perspective that endoscopic examination may replace vaginal cytology in determining the optimal insemination time of bitches or be used in addition to other methods, such as progesterone testing and vaginal cytology (Moxon et al., 2012).
Cytological method (vaginal cytology, vaginal swab examination)
Is one of the simplest methods of tracking the stage of the estrous cycle, and some authors consider it the most useful tool (Allen, 2002; Karnouhova, 2011b; Radohlib & Krajevs'kyj, 2014; Hahn et al., 2017; Grishina & Minjuk, 2016; Plemjashov et al., 2016; Stekol'nikov et al., 2018). Based on determining the ratio of different types of epithelial cells of the vagina and serves as an indicator of the hormonal background of the bitch (Tsutsui, 1975; Allen, 2002; Kim et al., 2005; Thomas, 2013; Plemjashov & Plahova, 2018; Djul'ger & Djul'ger, 2018). The method requires a lot of experience from the diagnostician and, although it gives an illustrative picture of all stages of the estrous cycle, it should be borne in mind that in exceptional cases, the maximum keratinization of the vaginal epithelium will be 10 days before ovulation or the day after (England & Concannon, 2002).
The increase in estradiol concentration during proestrus stimulates cell division in the basal layers of the vaginal epithelium, but then the estradiol concentration and, consequently, the endocrine support for the formation of new, stratified epithelium decreases, so more dead keratin cells are found in the sample. As proestrus develops, the number of epithelial cells containing the nucleus decreases. From the beginning to the middle of proestrus in vaginal smears find the increased content of erythrocytes. The peak of keratinization coincides with the beginning of the increase in the concentration of progesterone; however, at the beginning of estrus, the study does not reveal any characteristic features that could indicate the beginning of the fertile period. As a rule, this period comes a few days later (England & Concannon, 2002; Simpson et al., 2005; Hahn et al., 2017).
To obtain a sample using a swab, which is inserted into the vagina. The resulting smear is placed under a microscope and then stained with a contrast (trichrome) or non-contrast (eg, Diff-Quik) substance. With this staining, dead keratinized cells turn orange, while active nuclear cells, as well as basal and parabasal epithelial cells, take on different shades, from blue to green (Concannon & DiGregorio, 1986; Simpson et al., 2005).
Towards the end of estrus, vaginal discharge undergoes characteristic changes (epithelial cells containing the nucleus are rediscovered, and a large number of leukocytes appear). This pattern is usually seen 7-9 days after the peak of luteinizing hormone and is known as a "vaginal smear of metestrus" (at this time, more than 80% of epithelial cells are non-nuclear). Changes in the nature of secretions are preceded by a transition period, which is characterized by an increasing number of active cells and indicates the end of the fertile period and estrus (Allen, 2002; Koval'ov, 2003; Derkach, 2011; Grishina & Minjuk, 2016; Hahn et al., 2017; Djul'ger & Djul'ger, 2018).
Vaginal cytology is a practical, simple and inexpensive method that is very informative in terms of determining the phase of the estrous cycle (Bell et al., 1973; Tsutsui, 1975; Post, 1985; Allen, 2002; Karnouhova, 2011b; Bordjugov et al., 2013; Plemjashov et al., 2016; Stekol'nikov et al., 2018). However, significant differences in the time of onset of the main signs of estrus in relation to the peak of fertility limits the use of this technique. Thus, in some cases, smears during the entire period of fertility are likely to detect polymorphonuclear leukocytes, and in some bitches the peak number of non-nuclear cells is only 60% (Allen, 2002). Therefore, some authors consider the reliability of vaginal cytology to determine the period of fertility in bitches to be contradictory (Fontbon, 1999; Hiemstra et al., 2001; Lévy & Fontbonne, 2007; Moxon et al., 2010). Therefore, it is not recommended to determine the optimal mating time of bitches based on the study of only one vaginal swab (Arlt, 2018). However, it can be used to determine the stage of the cycle in general, for example, the early part of the follicular phase or metestrus and to detect disorders in the follicular phase (Hiemstra et al., 2001). Therefore, vaginal cytology due to its cheapness, simplicity and availability remains a popular method of determining the reproductive status of bitches (England & Concannon, 2002; Simpson et al., 2005).
Measurement of hormone concentration
It is known that during the estrous cycle there is a cascade of hormonal reactions. Thus, the level of estrogen in proestrus increases, and the level of luteinizing hormone and progesterone decrease. In the estrus phase, estrogen levels fall and progesterone levels begin to rise (Concannon et al., 1977; De Gier et al., 2006; Bordjugov et al., 2013; Hahn et al., 2017; Olğaç et al., 2017).
The concentration of progesterone in the plasma of the bitch on the estrous begins to gradually increase 2-3 days before ovulation and allows you to predict ovulation, confirm its presence and determine the period of fertilization (Johnston & Root, 1995; Allen, 2002; England & Concannon, 2002; Hahn et al., 2017; Djul'ger & Djul'ger, 2018).
Data on the progesterone concentration in the serum can serve as a guide for determining the fertile period in bitches (England & Concannon, 2002; Pibo & P'erson, 2002; Kustric & Dzhonston, 2005; Lévy & Fontbonne, 2007; Sridevi et al., 2012; Thomas, 2013; Davidenko & Ponomarenko, 2016). At the end of anestrus, the concentration of progesterone in the serum is at the basal level (and is practically undetectable), by the end of proestrus it decreases, but can be determined (about 3 nmol / l or 1 ng / ml). And continues to rise, reaching the level of 3-6 nmol / l (1-2 ng / ml) until the ovulatory peak of luteinizing hormone; 2 days later (on the day of ovulation) the indicators reach 6-12 nmol / l (2-4 ng / ml), and up to 4 days (ie before the beginning of the fertile period) - 18-30 nmol / l (6-10 ng / ml) (Allen, 2002; Simpson et al., 2005; Davidenko & Ponomarenko, 2016; Hahn et al., 2017; Djul'ger & Djul'ger, 2018; Hollinshead & Hanlon, 2019).
The concentration of progesterone is determined in blood plasma, because the analysis of other fluids (saliva and urine) currently does not give accurate results. The study is performed after the first signs of proestrus and repeated at least every 2-3 days until the end of proestrus or the beginning of behavioral estrus (Plemjashov & Plahova, 2018). This technique allows you to detect a rapid increase in the progesterone concentration before ovulation and accordingly calculate the date of possible mating. In cases of very short proestrus, studies begin earlier. Depending on the method of research (radioimmune or enzyme-linked immunosorbent assay), some differences in progesterone concentrations are possible; it should be borne in mind that the enzyme-linked immunosorbent assay gives overestimated, but otherwise reliable results (Simpson et al., 2005; Djul'ger & Djul'ger, 2018).
Ready-made enzyme-linked immunosorbent assay systems designed to determine the concentration of progesterone in blood plasma at different stages of the estrous cycle, give results that are almost twice the radioimmunoassay data, but at the same time reflect the characteristic cyclic changes (Kustric & Dzhonston, 2005). With the help of ready-made kits, it is not possible to obtain absolute indicators of progesterone concentration, but it is possible to trace relative changes in its concentration from the beginning of proestrus to the end of the cycle.
Serum progesterone tests are a good indicator of ovulation. Given the long period of sexual receptivity in bitches, including ovulation and gamete maturation, the interval between 4 and 7 days after the peak concentration of luteinizing hormone should be considered fertile (England & Concannon, 2002). However, it is possible that closer to the end of the fertile period, the ability to fertilize decreases, while some situations require special consideration - such as artificial insemination with frozen sperm (Wright, 1991; Lévy & Fontbonne, 2007; Steckler et al., 2013).
The concentration of progesterone determined by radioimmunoassay should be maintained at 30 nmol / l (10 ng / ml) on the first day and between 55 and 75 nmol / l (18-25 ng / ml) on the second day of insemination. The Enzyme-Linked ImmunoSorbent Assay (ELISA) method is also suitable for assessing the qualitative and quantitative content of progesterone (Simpson et al., 2005; Van Klaveren et al., 2001).
The high degree of reliability of determining the timing of ovulation allows not only to increase the percentage of successful inseminations, but also the fertility of the bitch - the more accurate the ovulation time, the more oocyte will meet with sperm and fertilization will occur (Bordjugov et al., 2013; Labib et al., 2018).
However, determining the level of progesterone in the blood is a costly and time-consuming method. The progesterone test does not determine exactly when ovulation occurred. For example, with the development of pyometra, the level of progesterone will be quite high, and vaginal discharge is often mistaken by owners for heat (Stekol'nikov et al., 2018).
Luteinizing hormone is a hormone that stimulates ovulation. It is released as one large peak, with high concentrations, persists for about 24 to 48 hours. The concentration of luteinizing hormone in the serum more than 1 ng / ml precedes ovulation for 2-3 days (Hahn et al., 2017). It is considered the optimal indicator for the diagnosis of ovulation, as it is predictable and reliable (Phemister et al., 1973; Wright, 1991; Renton et al., 1992; Nishiyama et al., 1999; Allen, 2002). But the problem is that surge of luteinizing hormone is short-lived, so sampling should be carried out frequently. In addition, test kits have a short shelf life (Thomas, 2013) and in general such research is quite valuable (Lévy & Fontbonne, 2007).
Plasma estrogen measurement has no advantage over luteinizing hormone levels due to its variable value in bitches and the rapid decrease in luteinizing hormone levels the day before the surge. During proestrus, developing ovarian follicles produce estrogen at 2-10 pg / ml for 10-14 days. Then the peak of estrogen reaches 50-120 pg / ml, approximately 2-3 days before estrus, followed by a rapid decrease immediately before the surge of luteinizing hormone (Simpson et al., 2005; Hahn et al., 2017). Developed enzyme immunological kits for rapid determination of the progesterone concentration and luteinizing hormone (LH ELISA) in the blood of dogs directly in the clinic were unsuitable for practical use due to insufficient accuracy of the method for measuring the concentration of progesterone in the blood and short-term preovulatory release of luteinizing hormone (Djul'ger & Djul'ger, 2018).
Ultrasound
It has been clearly demonstrated that bitch ovaries can be identified by real-time diagnostic ultrasound. Careful and repeated examination allows you to control the growth of follicles and detect the time of ovulation (England & Concannon, 2002; Eker & Salmanolu, 2006; Barbosa et al., 2013; Hahn et al., 2017; Olğaç et al., 2017). For its carrying out the device equipped with the transducer of 7,5-15 MHz is necessary (Wallace et al., 1992; Hayer et al., 1993; Bicudo et al., 2010; Bordjugov et al., 2013). The reference point for the uterus is the bladder, and for the ovaries the caudal poles of the kidneys of the corresponding side. In estrus, the endometrium thickens to 0.5-0.8 cm, the lumen of the uterine horns becomes visible and has a hypoechoic structure. Ovaries of irregular shape, have large (0.6-1.2 cm) anechoic structures (follicles) of round or oval shape with a thin wall (Yeager & Concannon, 1996; England & Concannon, 2002; Eker & Salmanolu, 2006; Inglend, 2006; England et al., 2009; Barbosa et al., 2013).
Unfortunately, in bitches during ovulation it is difficult to assess the ultrasound picture of the ovaries, in contrast to animals of other species. Thus, persistent follicles have a similar pattern before and after ovulation, and ovarian follicles and corpus luteum differ very poorly on ultrasound immediately before ovulation and for several days. Some ovulated follicles do not always completely collapse during ovulation and are gradually replaced by luteal tissue, while maintaining an echogenic pattern. In addition, non-ovulating follicles can complicate the interpretation of the ultrasound picture (England & Concannon, 2002; Plemjashov & Plahova, 2018). In this regard, it is recommended to perform at least two ultrasounds per day to finally accurately determine ovulation (Fedotov et al., 2014). In addition, it was shown that ultrasound performed daily in 20% of bitches compared with the quantitative content of progesterone alone has increased the accuracy of diagnosis of ovulation.
The bitch intended for ultrasound examination needs to have a haircut, which is also one of the inconveniences, but this problem can be solved by applying an amount of gel that will reduce the haircut area to a minimum, and often this is enough for dogs with short hair.
Ultrasound is currently one of the new classic methods of additional monitoring of ovarian status (Bicudo et al., 2010). This method allows to clarify the time of ovulation, mainly when using chilled or frozen semen of low quality due to the short time of its use directly during insemination, which should not be neglected. Compared with progesterone tests, the use of ultrasound to detect ovulation can increase accuracy.
In addition, some authors argue that one daily scan of the ovaries was sufficient to correctly detect the time of ovulation (Lévy & Fontbonne, 2007).
Bergeron et al. concluded that color Doppler ultrasound, performed once a day, was more accurate than ultrasound in B-mode, and allowed prospective determination of ovulation (Bergeron et al., 2013).
Ultrasound examination of the ovaries and Doppler measurement of the uterine and ovarian arteries can detect specific changes during the periovulatory phase. When considered together, measurements can be used to more accurately determine the date of ovulation, and these estimates should be performed consistently by an experienced professional (Marseloo et al., 2004; Barbosa et al., 2013; John & Thomas, 2015; Jurczak & Janowski, 2018).
However, some authors do not consider the method of ultrasound to be a priority in diagnosing the optimal insemination time in bitches, given the variability of image quality, the need for daily examinations and minor changes associated with ovulation, preferring hormonal methods for progesterone and luteinizing hormone (Renton et al., 1992; Silva et al., 1996; Thomas, 2013).
Nevertheless, ultrasonography is the method that most bypasses the limitations of practical implementation related to economic feasibility, technical complexity, practical capacity, objectivity, informativeness. In addition, only it allows you to determine the truth (morphological) signs of ovulation (Boyd et al., 1993). And such opportunities are becoming more real with the advent of the latest devices with increased capacity.
Other aspects of determining the fertile period
Method for determining the concentration of glucose in vaginal secretions. The content of sugars in cervical mucus is important for creating conditions for survival and preservation of sperm fertility. Lack of sugars (as well as excess chlorides and free calcium ions) reduces the negative electric charge and agglutination occurs (Torrans & Muni, 2006).
The technique can be useful in combination with vaginal cytology (England, 1992; England & Concannon, 2002). Therefore, in general, this method has not found wide practical application, because the meritoriousness of this technique has not been confirmed by scientific research (Allen, 2002; England & Concannon, 2002; Bordjugov et al., 2013; Thomas, 2013).
Temperature measurement method. The diagnostic value of this method is also low, because according to some authors, temperature rise in estrus is found in only 27% of bitches. Therefore, it can only be used as an auxiliary method (Bordjugov et al., 2013).
Infrared thermography is a promising non-contact remote diagnostic rapid method. Being absolutely harmless and objective method of inspection, allows to reveal differences in distribution and intensity of infrared radiation depending on physiological or pathological condition of an organism of animals. (Harper, 2000; Purohit, 2008; Stelletta et al., 2012; Rekant et al., 2016). In recent years, thermographic monitoring has become a useful technique and has yielded valuable results in animal reproduction. Among other things, the method allows to detect and differentiate the phenomena of the sexual cycle in females of different species (Sykes et al., 2012; Koshevoj et al., 2013; Luño et al., 2013; Simões et al., 2014; Talukder et al., 2014). The temperature gradient and color palette of the external genitalia are preventively determined, and then detailed studies are conducted in accordance with the guidelines and instructions for determining the optimal time of insemination. The temperature difference between the body and the surface of the vulva increases at the stages of proestrus and estrus. At the beginning of estrus, this difference is at its highest, and then decreases by the time of ovulation. The thermogram of the external genitalia of females in the stage of estrus is characterized by the predominance of "hotter" colors of the palette (red), and in the stage of metestrus - warm (orange).
Thus, the results of research reliably confirm the regularity of increasing the temperature of the external genitalia in females in the stage of estrus, which can be defined remotely as a method of preventive (contactless, remote-project) diagnosis of sexual cycle phenomena (Koshevoj et al., 2013).
Olğaç et al. conducted research to evaluate the effectiveness of thermographic monitoring to identify stages of the estrous cycle and ovulation time in bitches (Olğaç et al., 2017). The authors report that the use of this method reduces the cost and time to detect estrus. However, the reliability and accuracy of thermographic monitoring techniques can be improved due to more research in an isolated environment from different external conditions (Olğaç et al., 2017).
Therefore, thermographic monitoring alone is not enough to detect ovulation time, however, it can be used as an adjunct to other modern methods (Koshevoj et al., 2013; Olğaç et al., 2017).
Recent contrast-enhanced radiographs of the vagina have shown a strong association between estradiol and progesterone blood levels and cervical dilatation. The results of these studies are crucial for determining the fertile period in dogs, as the cervix remains closed (impermeable to contrast medium and probably sperm) until there is a decrease in estradiol levels and an increase in the progesterone / estradiol ratio during the preovulatory period. After the peak of luteinizing hormone, the cervix remains open for about 6 days (ie it closes about 2 days before the onset of cytological metestrus), and during this period the concentration of progesterone remains high, and estradiol is virtually undetectable. These data suggest that sperm have access to the cervix only for a limited time of estrus, contrary to popular belief that mating can be performed both before and after opening the cervix. Changes in the condition of the cervix are explained by the thickening of the mucous membrane under the influence of estrogen at the stage of proestrus, which leads to closure of the cervix. Further opening of the cervix is accompanied by a decrease in swelling of the mucous membrane and due to an increase in the ratio of progesterone / estradiol (Simpson et al., 2005).
Interestingly, the period when the cervix is open, corresponds to the optimal timing of mating, calculated on the basis of the results of endoscopic examination of the vaginal mucosa. Contrast-enhanced radiographic findings are easy to interpret, but do not explain how blood and uterine secretions penetrate the swollen cervix during the proestrus stage. Apparently, the effects of other activators, such as estrogen, which can relax smooth muscles, as well as prostaglandins contained in semen. The need for luteinizing hormone indicates that persistent manifestations of heat are observed only immediately before the fertile period (England & Concannon, 2002). Although the onset of heat is an individual characteristic of the each animal in relation to the peak of luteinizing hormone, these manifestations can serve as a guide for calculating the fertile period (Simpson et al., 2005).
Bergeron et al. indicate that all the methods they evaluated (vaginal impedometry, analysis of serum progesterone concentration, vaginoscopy and vaginal cytological evaluation) often gave inaccurate results when used individually. Therefore, the authors conclude that several methods should be used to determine the optimal insemination time of bitches (Bergeron et al., 2014).
In order to minimize the cost of research to determine the optimal insemination time of bitches, some believe that it is sufficient to conduct only vaginal cytology and analysis of hormones - luteinizing hormone, progesterone or estradiol (Linde & Karlsson, 1984; Wright PJ. 1990; Bouchard et al., 1991; Badinand et al., 1993; Groppetti et al., 2015; Hahn et al., 2017; Olğaç et al., 2017; Stekol'nikov et al., 2018).
Other authors believe that the detection of ovulation and insemination in bitches should be based on hormone concentrations, vaginal cytology and endoscopic examination of the vagina (Okkens et al., 1985; Jeffcoate & Lindsay, 1989; Rao et al., 2011).
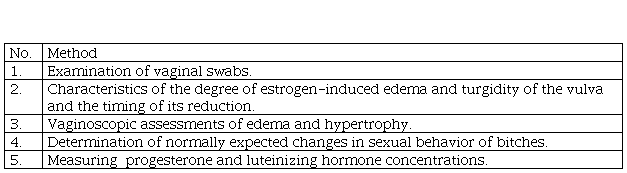
According to England and Concannon, the protocol for determining the optimal insemination time of bitches should include:
examination of vaginal swabs taken every 1-3 days to monitor the degree, percentage or index of keratinization of vaginal epithelial cells, the presence and absence of leukocytes in the smear;
characteristics of the degree of estrogen-induced edema and turgidity of the vulva and the timing of its reduction associated with periovulatory estrogen decline;
vaginoscopic assessments of edema and hypertrophy caused by increased estrogen levels, timing and extent of further reduction of vaginal mucosa and folds caused by decreased estrogen levels shortly before and after ovulation;
determination of normally expected changes in sexual behavior of bitches, including the timing and degree of sexual susceptibility during the normal periovulatory period;
measuring serum or plasma progesterone concentrations and estimating the day of luteinizing hormone surge as the time when progesterone most likely first exceeded 1-2 ng / ml (England & Concannon, 2002).
Based on the above, for successful mating is necessary:
to combine research of vaginal smears and measurement blood progesterone level;
the first vaginal examination should be performed 3-5 days after the onset of heat with an interval of 3-5 days;
if more than 60% of keratin cells are detected in the smear, start measuring progesterone with an interval of 1-2-3 days.
Kustric & Dzhonston believe that the main recommendations for determining the fertile period in bitches are as follows:
the beginning of taking cytological samples from the vagina for 4-5 days after the onset of proestrus;
-
when the number of keratinized vaginal epithelial cells exceeds 80% of all exfoliated cells, blood samples should be taken to determine serum progesterone levels;
when the number of keratinized vaginal epithelial cells exceeds 80% of all exfoliated cells, blood samples should be taken to determine serum progesterone levels;
it is recommended to take blood samples to determine the serum concentration of progesterone every 2-3 days until the concentration begins to increase, which will be an indicator of the release of luteinizing hormone or ovulation. This will allow to predict the optimal period for fertilization. If serum progesterone concentration is measured by ELISA, samples should be taken daily until the concentration reaches higher values (5.0-10.0 ng / ml; 15.5-31.0 nmol / l);
the probability of conception increases for mating in the period from the 4th day before to the 2nd day after ovulation, and the maximum litter can be obtained from mating on the 2nd day after ovulation (Kustric & Dzhonston, 2005).
However, all these methods involve determining the indirect signs of the optimal insemination time. Therefore, there is currently no standard method for assessing ovulation and the optimal insemination timing of bitches (Hase et al., 2000; De Gier et al., 2006; Togoe et al., 2014).
The most unified is the scheme, which should provide (on condition that the animal is clinically healthy, has no abnormalities of the sexual cycle in the anamnesis):
cytological examination of vaginal smears - is carried out for 3-4 days from the moment of detection of heat signs by the owner
repeated examination (doctor prescribes a day) of vaginal smears to monitor the dynamics of cytological changes in the vaginal mucosa;
ultrasound diagnosis of follicles in the ovaries. According to the results of the above studies (at the discretion of the doctor) blood sampling to determine the concentration of progesterone;
control ultrasound examination of the ovaries to determine the dynamics of follicle development;
re-analysis of the progesterone concentration in the blood to determine the most accurate day of ovulation and mating.
Conclusion
The period of fertilization or the time when the oocyte can be fertilized is 2-5 days after ovulation. Therefore, mating should be performed immediately before or during this period. Determine the optimal mating time using various methods that determine the period of fertility (when mating can lead to conception), and preferably during fertilization However, none of the diagnostic methods is absolutely reliable, so for the most reliable result, it is recommended to use several (two or three) studies. For example: counting days, assessment of vulvar and vaginal discharge plus smear and (or) vaginoscopy Control mating increases the probability of conception and is usually performed the day after the first mating. It should be remembered that the time of ovulation in the same bitch during different heat can be different. For bitches with problems of conception use the maximum number of methods with the analysis of past successful and unsuccessful matings.