Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Kurtziana
versión On-line ISSN 1852-5962
Kurtziana v.34 n.1-2 Córdoba ene./dic. 2009
Medicinal plants: A general review and a phytochemical and ethnopharmacological screening of the native Argentine Flora
Gloria E. Barboza1, 3, 4, Juan J. Cantero2, César Núñez2, Adriana Pacciaroni1, 3 & Luis Ariza Espinar1
1 Instituto Multidisciplinario de Biología Vegetal (IMBIV-CONICET), Casilla de Correo 495, 5000, Córdoba, Argentina.
2 Departamento Biología Agrícola, Facultad de Agronomía y Veterinaria, Universidad Nacional de Río Cuarto, Ruta Nac. 36 - Km. 601 - Código Postal X5804BYA, Río Cuarto, Córdoba, Argentina.
3 Facultad de Ciencias Químicas, Universidad Nacional de Córdoba, Avda. Haya de la Torre y M. Allende s.n., Córdoba, Argentina.
4 Autor para la correspondencia: gbarboza@imbiv.unc.edu.ar.
Summary
Barboza, G. E., J. J. Cantero, C. Núñez, A. Pacciaroni & L. Ariza Espinar. 2009. Medicinal Plants: A general review and a phytochemical and ethnopharmacological screening of the native Argentine Flora. Kurtziana 34 (1-2): 7-365.
A review and a checklist based on empirical evidence of the therapeutic properties of the native medicinal flora from Argentina are presented. The chemical constituents and biological activity of each species, when known, are also provided. Medicinal flora comprises 1,529 taxa of vascular plants (Pteridophyta: 56; Gymnospermae: 13; Monocotyledoneae: 152; Dicotyledoneae: 1,308), including 115 endemic species. In addition, the distribution of these species is analyzed, and the endemic areas are also stated.
Key words: Flora medicinal; Argentina; Review; Folk medicine; Diversity; Endemisms.
Resumen
Barboza, G. E., J. J. Cantero, C. Núñez, A. Pacciaroni & L. Ariza Espinar. 2009. Plantas medicinales: Revisión y "screening" fitoquímico y etnofarmacológico de la flora nativa de Argentina. Kurtziana 34 (1-2): 7-365.
Se presenta una revisión y se provee un catálogo de la flora medicinal nativa de Argentina basado en el conocimiento empírico de las propiedades terapéuticas de las especies. Se complementa con la composición química y actividad biológica para cada especie cuando la información está disponible. Se reportan 1529 taxones medicinales de plantas vasculares (Pteridófitas: 56; Gimnospermas: 13; Monocotiledóneas: 152; Dicotiledóneas: 1308), incluidas 115 especies endémicas. Además, se realiza un análisis de la distribución de estas especies y se determinan las áreas de endemismos.
Palabras clave: Flora medicinal; Argentina; Revisión; Medicina popular; Diversidad; Endemismos.
Introduction
Plants have provided man with all his needs in terms of shelter, clothing, food, flavours and fragrances as not the least, medicines. Plants have formed the basis of sophisticated Traditional Medicine (TM) systems that have been in existence for thousands of years and continue to provide mankind with new remedies. Some of the oldest known medicinal systems of the world such as Ayurveda of the Indus civilization, Arabian medicine of Mesopotamia, Chinese and Tibetan medicine of the Yellow River civilization of China and Kempo of the Japanese are all based mostly on plants. The ancient cultures are known for their systematic collection of information on herbs and their rich and well-defined herbal pharmacopoeias. Although some of the therapeutic properties attributed to plants have proven to be erroneous, medicinal plant therapy is based on the empirical findings of hundreds and thousands of years (Gurib Fakim, 2006).
According to OPS (Arias, 1999) a medicinal plant is (1) any plant used in order to relieve, prevent or cure a disease or to alter physiological and pathological process, or (2) any plant employed as a source of drugs or their precursors. A phytopharmaceutical preparation or herbal medicine is any manufactured medicine obtained exclusively from plants (aerial and non-aerial parts, juices, resins and oil), either in the crude state or as a pharmaceutical formulation (Rates, 2001).
There is ample archaeological evidence indicating that medicinal plants were regularly employed by people in prehistoric times. In several ancient cultures botanical products were ingested for biomedically curative and psychotherapeutic purposes (Halsberstein, 2005). Knowledge of medicinal plants has usually resulted from trial and error methods, and often based on speculation and superstition (Hamayun et al., 2006). The strong historic bond between plants and human health began to unwind in 1897, when Friedrich Bayer and Co. introduced synthetic acetyl salicylic acid (aspirin) to the world. Aspirin is a safer synthetic analogue of salicylic acid, an active ingredient of willow bark, and was discovered independently by residents of both the New and Old worlds as a remedy for aches and fevers (Raskin et al., 2002). Medicinal plants have contributed to humanity's health care, source of livelihood cultural traditions, and financial gains, among others (Hamilton, 2004). However, medicinal plants are constrained by procedures such as classification, identification, and characterization.
Some 80% of the world's population still relies upon plants for primary health care; even today in Western medicine, and despite progress in synthetic chemistry, some 25% of prescription medicines are still derived either directly or indirectly from plants (Farnsworth & Soejarto, 1991). Nearly 50,000 species of higher plants have been used for medicinal purposes. They are also used in food, cleaning, personal care and perfumery. In systems of traditional healing, major pharmaceutical drugs have been either derived from or patterned after compounds from biological diversity (Bisset, 1994). A trend in phytomedicine is the use of new plant origin bioactive compounds with the potential for chemical modification, which will broaden phytomedical importance. Molecular biology is also being used in this process and the pharmacological profiles of these compounds are screened using new research equipment and new technology (Cordell, 2000; Cordell & Colvard, 2005; Yaniv & Bachrach, 2005; Pieters & Vlietinck, 2005).
The use of plants in medicines ranges from crude preparations or extracts, to refined extracts and single molecular species. In terms of categories of use this encompasses food supplements, herbal medicines, botanical drugs and prescription medicines. Increased interest in plants as a source of novel pharmacophores recognizes their chemical diversity and versatility, not matched by synthetic chemistry libraries. In spite of the surge of activity in synthetic chemistry over the last 20 years or so, almost half the some 850 small molecules introduced as drugs were derived from plant sources. Over 100 small molecules derived either directly or indirectly from plants are currently at some point in the clinical trials process (Fowler, 2006).
The beneficial medicinal effects of plant materials typically result from the combinations of secondary products present in the plant. That the medicinal actions of plants are unique to particular plant species or groups is consistent with this concept as the combinations of secondary products in a particular plant are often taxonomically distinct (Kaufman et al., 1999). Ecological function of secondary products may have some bearing on potential medicinal effects for humans. For example, secondary products involved in plant defense through cytotoxicity toward microbial pathogens could prove useful as antimicrobial medicines in humans, if not too toxic (Briskin, 2000). In contrast to synthetic pharmaceuticals based upon single chemicals, many phytomedicines exert their beneficial effects through the additive or synergistic action of several chemical compounds acting at single or multiple target sites associated with a physiological process. This synergistic or additive pharmacological effect can be beneficial by eliminating the problematic side effects associated with the predominance of a single xenobiotic compound in the body (Tyler, 1999).
Ethnobotanical studies have become increasingly valuable in the development of health care and conservation programs in different parts of the world. The green pharmaceuticals are receiving extraordinary importance and popularity. Ethnobotany and ethnopharmacology have contributed to the discovery of many important plant-derived drugs. Soejarto et al. (2005) claimed that future mass bioprospecting effort must incorporate important consideration about team scientific expertise (of all relevant disciplines) together with expertise in a wide range of human endeavors, including diplomacy, international laws and legal understandings, social sciences, politics, anthropology and good common sense.
Development of medicinal plants relies very heavily on the knowledge carried by indigenous peoples and rural societies. Herrera Vasquez & Rodríguez Yunta (2004) argued the importance of taking account of indigenous knowledge or «ethnoknowledge».
The pharmaceutical and biotechnological industries are much interested in using this knowledge for the discovery, development and application, within biodiversity, of new active products on health and new genes with properties for food improvement (Heinrich & Gibbons, 2001). In recent years, the plant remedies used in TM - both in traditional herbal medicine and in shamanic healing-have received considerable attention for ethnobotanist, and today the chemistry and pharmacology of many of these are well understood (Cotton, 1997). Chemical analyses and biological assays have begun to play an important part in ethnobotanical studies and there are now numerous examples where scientific analyses have provided objective evidence to validate traditional plant use, for example Homalanthus nutans (G. Forst.) Guill. (Euphorbiaceae), used by samoan healers against the viral disease yellow fever; extracts have been found to exhibit potent antiviral activity, particularly against the human immunodeficiency virus HIV-1 (Balick & Cox, 1996). As new uses of medicinal plants are discovered and popularized, the concern for sustainability is being increasingly addressed; concern over the growth in biopiracy also combines with the critical need for the conservation of species and their habitat (Science Reference Services, 2008).
The search for new molecules, nowadays, has taken a slightly different route where the science of ethnobotany and ethnopharmacognosy are being used as guide to lead the chemist towards different sources and classes of compounds (Gurib-Fakim, 2006). Plant derived natural products hold great promise for discovery and development of new pharmaceuticals (McChesney et al., 2007).
Since ancient times, medicinal plants have been harvested from the wild (Mshigeni et al., 1991; Balick & Cox, 1996; Sheldon et al., 1997; Dhillion & Ampornpan, 2000; Singh & Padmalatha, 2004). In many rural communities, traditional medicine (TM) is still viewed as the mainstay of primary health care systems (Bannerman et al., 1983; Manandhar, 1994; Svarstad & Dhillion, 2000; Manandhar, 2002) due to its effectiveness, cultural preference or absence of «modern» alternatives (Plotkin & Famolare, 1992; Taylor et al., 1995; Balick et al., 1996; Tabuti et al., 2003).
TM is a comprehensive term which describes medical knowledge systems such as the traditional Chinese, the Indian ayurveda, the Arabic unani, or other forms of indigenous or folk herbal medicine. In countries where the prevailing health care system is largely dependent on allopathic medicine, or where TM has not yet been incorporated to meet national health care needs, TM is often referred to as «complementary», «alternative» or «nonconventional» (WHO, 2002).
It is well established that this type of health knowledge and practice plays a fundamental role in the health programs of developing countries. Indeed, for centuries TM has been in certain communities the unique health care system available for the prevention and treatment of diseases. In addition, there has been an increasing awareness and understanding of the potential of herbal knowledge for discovering newer treatments, and for gaining insights into the socioeconomic, conservationist and cultural components of society (Alves & Rosa, 2007).
Nonbiomedical healing systems vary across cultures but conceptually they often focus on balance and harmony, which may be treated mentally, physically, or spiritually (Bodeker 1994; Hewson, 1998; O'Connor, 1998), that is they favor a «holistic» approach aiming at a systemic model where multiple biological, psychological and social dimensions are interlinked, often in combination with the patient's active participation in their own healing and recovery (McGaw et al., 2005). Biomedical treatment systems are often conceptually different than traditional systems focusing on Cartesian dualism, where body and mind are treated separately and technological and surgical procedures are often used for the treatment of physical diseases (Bodeker, 1994; Hewson, 1998).
There is a worldwide trend of increasing demand for many popular, effective species in Europe, North America and Asia, growing between 8 and 15% per year (Grünwald & Büttel 1996). In Japan, 60-70% of allopathic doctors prescribe TM for their patients, and in China, TM accounts for about 40% of all health care. Forty-eight percent of the populations in Australia, 70% in Canada, 42% in the US, 38% in Belgium and 75% in France, have used TM at least once. In the United Kingdom, almost 40% of all general allopathic practitioners offer some form of TM referral or access (Bussmann & Sharon, 2006). A similar situation exists in Latin America, where large volumes of medicinal plants are sold in urban markets (Shanley & Luz, 2003). Regional Office for the Americas (AMRO/PAHO) reports that 71% of the populations in Chile and 40% of the population in Colombia use TM.
Current research in drug discovery from medicinal plants involves a multifaceted approach combining botanical, phytochemical, biological, and molecular techniques. In order to understand the biological activity of a plant, be it medicinal, poisonous, or nutritive, it is necessary to know its chemical constituents. Thus, they are plant secondary and primary metabolites (e.g. alkaloids, terpenoids, phenolics, gums, mucilages, carbohydrates, amino acids, proteins, fatty acids, glycolipids, etc.) that organize medicinal plants (Croteau et al., 2000).
Knowledge of plant bioactivity has been accumulated by experimentation over centuries by people living in intimate association with their environment. Therefore, ethno-directed research is very useful in drug discovery and development (Cox & Balick, 1994; Heinrich & Gibbons, 2001). However, accelerated acculturation is disintegrating ethnopharmacological information often faster in many areas than the extinction of plant species, which rampant deforestation invariably entails (Sanz-Biset et al., 2009). This problem is particularly serious in the tropical rainforests (Plotkin & Famolare, 1992). Soejarto & Farnsworth (1989) stress the special significance of these tropical areas as sources of new pharmaceutical agents. Mendelsohn & Balick (1995) conclude that only one-eighth of all drugs that can be potentially developed from the rainforests of the world, have been discovered. Only the Amazon has approximately 16% of all the plant species that exist today on the Earth, and this wealth increases towards the west of the region (Schultes & Raffauf, 1990; Sanz-Biset et al., 2009).
The use of plants in the practice of medicine represents one of the biggest human use of the natural world. There is no reliable figure that represents the total number of medicinal plants on Earth, and national or regional estimations vary considerably (Schippmann et al., 2002). Estimates of species used therapeutically are included in the following Table:
TABLE 1 Number and percentage of medicinal species documented in different countries and regions
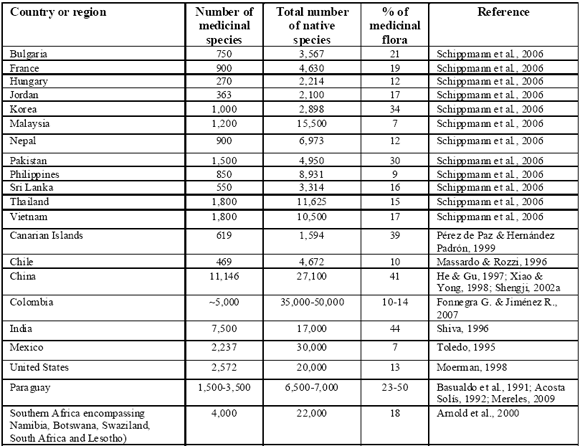
TM systems employ relatively few species: 500-600 commonly found in Traditional Chinese Medicine (Shengji, 2001); 1,430 in Mongolian Medicine (Shengji, 2002b); 1,1003,600 in Tibetan Medicine (Shengji, 2001, 2002b); 1,250-1,400 in Ayurveda (Dev, 1999); 342 in Unani; and 328 in Siddha (Shiva, 1996). Bioprospecting as the search for value in the biological world is an incredibly ancient practice. In a general way, any time that a person searches for food or other biological value in their enviroment, they are bioprospecting (McClatchey, 2005). Bioprospecting refers herein to the search for unknown therapeutic compounds found in organisms. However, this notion may also imply appropriation of legal entitlements on indigenous, especially biomedical knowledge of natural resources. The 1992 Convention on Biological Diversity (CBD) establishes sovereign national rights over biological resources and commits member countries to conserve them, share the benefits resulting from their sustainable use, i.e., finding new drugs, crops, and industrial products, while conserving the resources for future research. The World Trade Organization (WTO), by contrast, recognizes ownership of technology only, rather than knowledge.
Problems associated with biopiracy or excessive restrictions on research have come to assume policy prominence in the general thematic area of 'medicinal plant conservation and use'. The fair and equitable sharing of benefits from bioprospecting is required under the Convention on Biological Diversity, but it is not always easy to achieve these ideals in practice. While experience is accumulated in how this may practically be achieved, it is important, at the present time, that controls imposed on scientific research to prevent biopiracy or theft of local and indigenous intellectual property do not unduly restrict research that has little or nothing to do with these matters (Hamilton, 2004).
World trade in medicinal plants accounts for about 30 percent of the total drug market. This percentage excludes plants used as raw material sources for the essential oils required to manufacture cosmetics, food additives and for other non-medicinal purposes (Addae-Mensah, 2000). Most of these medicinal plants come from less industrialized countries that supply them as cheap raw materials to the multinational pharmaceutical manufacturers in the more industrialized countries. Of 76 compounds obtained from higher plants that are present in US prescriptions, only 7 percent are commercially produced by total synthesis (Farnsworth & Soerjarto, 1991). In 1976, imports of medicinal plants worldwide were estimated to be $US 355 million. This rose to $US 551 million by 1980 - an increase of over 60 percent within four years (Principe, 1989). But when these medicinal plants are processed into suitable dosage forms as safe and efficacious drugs, they are sold to the public at prices far beyond the affordable range for the majority of people in the relatively poor countries. Yet it is the botanic resources of these same countries that make possible the manufacture of these drugs for commercial markets (Addae-Mensah, 2000). Of the drugs prescribed in developed countries, 25% derives from about 100 plant species (Comer & Debus, 1996). Natural products also provide the scaffold molecules to synthesize further molecules (Miller & Brewer, 1992; Grifo et al., 1997). The economic impact of medicinal plants on the structure of local and national policies is by no means negligible. In the late 1990s, the annual market value of herbal drugs used worldwide was estimated to be around US $45 billion. Today, their value ranges from US $60 billion to US $100 billion (Shasany et al., 2007).
Sustainable use of wild populations of medicinal plants requires robust assessment of the distribution and abundance of target species. While it is increasingly recognized that sustainable harvest of wild populations "is one of the most misunderstood and misused concepts in today's conservation arena" (Struhsaker, 1998), and that sustainable use has no direct connection with the more encompassing concept of 'ecological sustainability' (Hall & Bawa, 1993), in practical terms there are often limited alternative options available to resource managers, especially given the long history of dependence of rural communities on harvesting from natural habitats, whether officially sanctioned or not. Ex situ cultivation and farming of target resource species is the most preferable option (Cunningham, 1994; Nantel et al., 1996; Tran et al., 2001; Pfab & Scholes, 2004).
The diversity of life on Earth is dramatically affected by human alterations of ecosystems and the reverse is also true: biodiversity in the broad sense affects the properties of ecosystems and, therefore, the benefits that humans obtain from them (Díaz et al., 2006). In general people who rely most directly on ecosystem services, such as subsistence farmers, the rural poor, and traditional societies, face the most serious and immediate risks from biodiversity loss and this is a general situation that happen with medicinal plants (Mertz et al., 2007).
The analysis of extensive databases has become an important tool evaluating the knowledge and conservation status of the biodiversity. A recent assessment (Bramwell, 2003) estimates that 21% of the world's flora is threatened. If the latter figure is applied to previously earlier extrapolation that 72,000 plant species are used medicinally, it leads us to estimate that about 15,000 medicinal plant species are threatened at least to some degree. In Argentina there is no evaluation between species global richness and the occurrence of threatened species (Villamil, 2006), only data from Buenos Aires province exist (Delucchi, 2006). In this province 369 of vascular plants has been reported as threatened from which 71 has medicinal uses.
In different countries, many medicinal plants are widely distributed and used across regions. However, relatively few are cultivated. Thus, the conservation of these plants requires that efforts are directed to key habitats, including secondary forests, disturbed areas and agrolandscapes (Aguilar-Støen & Moe, 2007). The majority of companies, the mass-market, over-the-counter pharmaceutical companies as well as the larger herb companies, prefer cultivated material, particularly since cultivated material can be certified 'biodynamic' or 'organic' (Laird & Pierce, 2002). Nevertheless, the number of medicinal plant species currently in formal cultivation for commercial production does not exceed a few hundred worldwide - less than 1% of the total number of medicinal plants used. One explanation why are so few species cultivated may be found in the observation that cultivated plants are sometimes considered qualitatively inferior when compared with wild-gathered specimens (Schippmann et al., 2006). Scientific studies partly support this. Medicinal properties in plants are mainly due to the presence of secondary metabolites which the plants need in their natural environments under particular conditions of stress and competition and which perhaps would not be expressed under monoculture conditions. Active-ingredient levels can be much lower in fast-growing cultivated stocks, whereas wild populations can be older due to slow growth rates and can have higher levels of active ingredients (Schippmann et al., 2006).
Cultivation of medicinal plants is widely viewed not only as a means for meeting current and future demands for large-volume production of plant-based drugs and herbal remedies, but also as a means for relieving harvest pressure on wild populations (Palevitch 1991; IUCN 1993; Lambert et al. 1997).
Booming markets with rapidly rising demands often have devastating effects on wild-collected species. But for example, if the seven forms of rarity described by Rabinowitz (1981) is taken in account it is clear that not all species are affected in the same way by harvesting pressures: a species which (i) has a narrow geographic distribution, (ii) is habitat-specific, and (iii) has small population sizes everywhere, is more easily over-harvested than species of any other pattern. Additionally susceptibility or resilience to collection pressure varies among species owing to biological characters such as different growth rates (slow-growing vs. fast-growing), reproductive systems (vegetative or generative propagation; germination rates; dormance; apomixis) and life forms. Species can be distinguished quite well in their susceptibility to over-collection if their life form and the plant parts collected are viewed together (Table 2). Species most susceptible to overharvest are habitat-specific, slow-growing and destructively harvested for their bark, roots or the whole plant. Patterns of medicinal plant use by local peoples are considered to vary as a function of plant habitat collection, cultural changes, and ecological and biochemical aspects (Albuquerque, 2006).
TABLE 2 Susceptibility of medicinal species to overcollection as a function of life form and plant parts used (adapted from Schippmann et al., 2006).

Many symposiums were organized to coordinate policies for conservation regulation management and rigorous scientific research to enhance and sustain global medicines from natural resources (Chaves, 2001; Arnason et al., 2005; Blacpma, 2006, 2007). For example, the WHO/IUCN/WWF Guidelines on the Conservation of Medicinal Plants (IUCN, 1993) provided a framework for the development of national strategies on the conservation and sustainable use of medicinal plants and put medicinal plant conservation on the international agenda for a diverse set of stakeholders, including national health authorities, environmental and trade authorities, conservation groups and policy makers. The Guidelines constituted an action plan for the development of IUCN Medicinal Plant Specialist Group, founded in 1994. Nowadays new documents have been prepared (Leaman & Salvador, 2005) referring these guidelines.
Many important thematic areas of medicinal plants have been covered by recently published handbooks: global perspective of medicinal plants, the evolution of herbal drugs with civilization, chemical-related information, actual uses (e.g. cathartic, emetic, carminative, stimulant, antihelmitic, expectorant, etc.), specific actions (e.g. anti-mitotic activity or pupil constriction, etc.), molecular biological activity under the main chemical headings (e.g. volatile oils inhibit cancer cells or act on the central Nervous System), topics such as antioxidant therapy or aromatherapy, technologies in plant research (e.g. production, in vitro cultivation, breeding, etc.), latest developments in medicinal applications, hopes, legal issues, conservation, ethnobotany, challenges connected with the use of medicinal plants (e.g. phytomedicine and biodiversity, quality control, clinical use and ethnopharmacy, etc.) and commercialization (Pengelly, 2004; Packer, 2004; van Wyk & Wink, 2004; Ross, 2005; Yaniv & Bachrach, 2005; Zhang & Demain, 2005; Daniel, 2006; Ramawat & Merillo, 2008; Ramawat, 2008; Garrido, 2008; Dewick, 2009).
In last years many contributions were also made dealing with macroregions (Roth & Lindorf, 2002; Gurib-Fakim & Brendler, 2004; Wiart, 2006; Khare, 2007), countries (Watanabe et al., 2005; Brouwer et al., 2005; González Torres, 2005; Libman et al., 2006; Pin & Céspedes, 2009), special sites and taxonomic groups (Addae-Mensah, 2000; Kinghorn, 2002; Tene et al., 2007; Kultur, 2007; Teklehaymanot & Giday, 2007; Upadhyay et al. 2007; Au et al., 2008; Lulekal et al., 2008; Sanz-Biset at al., 2009), affections (Kumara et al., 2007; Adams et al., 2007), health effects of chemical compounds (Okuda, 2005), ethnic groups (Coe, 2008), specific practices (Ticktina & Paule Dalleb, 2005; Mohagheghzadeh et al., 2006) and resources to document, conserve and disseminate ancient knowledge (Gaikwad et al., 2008). Halberstein (2005) reported an extensive bibliography on herbal interventions: 202 books ranging from 1847 to 2005 that reflect the profuse studies made in this field.
A big progress on the study of medicinal plants has been made in Latin America in the last years (Calixto, 2005). Nevertheless, complete catalogs of native medicinal species are still missing for many countries.
Argentina is the most extensive temperate country in South America (Fig. 1). A large part of its biodiversity can be attributed to the fact that in this territory different biogeographical regions converge (Zuloaga et al. 1999, 2008). The wide expanse of its continental area (2,791,810 km²), as well as its varied topographical, climatic and vegetational features, are essential to the formation of one of the most diverse flora in the southern Neotropics. Argentina is bordered by Uruguay, Brazil and the Atlantic Ocean to the east, Paraguay and Bolivia to the north, and Chile to the west (Fig. 1). The country is characterized by a wide range of macrohabitats across elevation and climatic gradients, from 7,000 m to the coastal plains and from subtropical to polar climates. Among these macrohabitats we can find the phytogeographic regions of Patagonia, Chaco, Altoandina, and Aconquija (Davis et al., 1997).
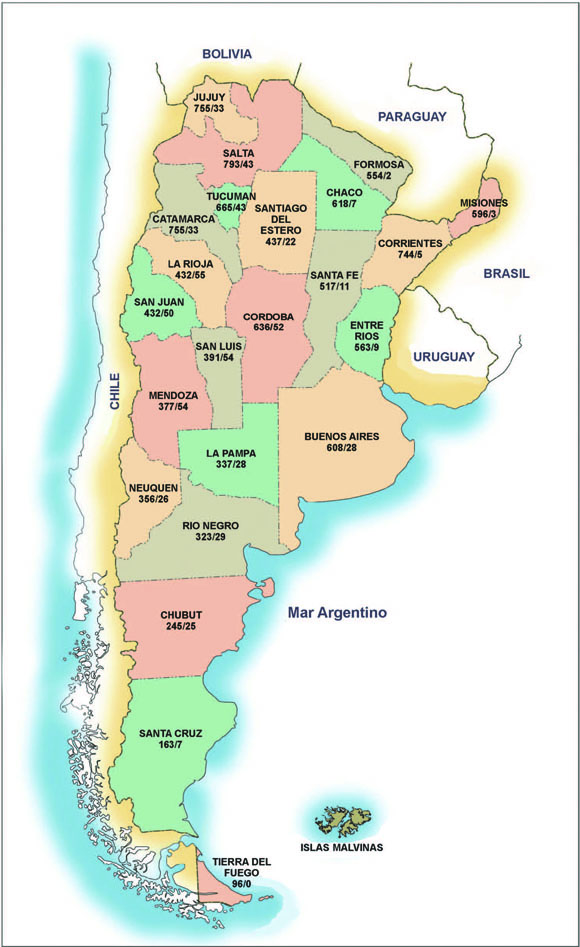
Fig. 1.- Argentina: total and endemics medicinal species respectively in each province
Argentina is also one of the 25 most diverse countries in the world due to their species richness and endemism (Caldecott et al., 1996; Zuloaga et al., 2008). In this country, the Northeast and Northwest regions have the highest number of families, genera and species, Misiones and Salta being the most important provinces (Zuloaga et al., 1999). In a recent analysis of the taxonomic diversity of the Argentine Dicots, Juárez et al. (2006) found that diversity of this group could not be explained by the area of provinces where they lived, but it varied through latitudinal and longitudinal gradients. They showed that the taxonomic diversity of these plants increases from high to low latitudes and west-east longitudes, and that main diversity centers are located in the Northeast region of this country. The high diversity of the Northwest provinces (e.i. Salta, Jujuy, Tucumán) was explained by the latitudinal gradient: this could be due to that tropical eco-regions have their limits of distribution in this region of Argentina, which added to the others eco-regions generate a high environmental heterogeneity (Juárez et al., 2006).
With a rich endemic flora, Argentina has 9938 species (Zuloaga et al., 2008), distributed in 274 families. Hence, the potential for developing pharmaceutical bioprospecting schemes is considerably high, particularly in ethnopharmacology. From an ethnobotanical point of view, the Montenegro's Materia Médica (1702) was the first contribution about the medicinal plants from Paraguay and Misiones (Quintana, 1945) but Plantae Diaphoricae (Hieronymus, 1882) was the first scientific treatment and significant bibliographical work in medicinal plant studies in Argentina; since then, research on folk medicine has provided valuable information from different regions. Detailed compendia of medicinal species native to or naturalized in Argentina include the following: Domínguez, 1928; Sorarú & Bandoni, 1978; Ratera & Ratera, 1980; Toursarkissian, 1980; Amorín, 1980-1981; Amorín & Rossow, 1989-1992; Gupta, 1995; Marzocca, 1997; Rondina et al., 2003; Alonso & Desmarchelier, 2006. On a regional scale, many areas have been deeply studied in relation to their traditional medicine, the following contributions are worth mentioning: Martínez Crovetto, 1964, 1965, 1968a, b, 1981; Arenas, 1981, 1983, 2000, 2003; Novara, 1984; Perez de Nucci, 1988; Xifreda, 1992; Filipov, 1994, 1997; Lahitte & Hurrell, 1996; Del Vitto et al., 1997, Bocco et al., 1997; Pochettino et al., 1997; Amat & Yajía, 1998, Méndez, 1998; Pochettino & Martínez, 1998; Vignale, 1996, 1998; Freire, 1998; Gratti, 1998; Lahitte et al., 1998; Hilgert, 2001; Núñez & Cantero, 2000; Scarpa, 2002, 2004; Martínez, 2002, 2007a, b, 2008a, b; Arbo & Tressens, 2002; Kutschker et al., 2002; Roig, 2002; Martínez & Planchuelo, 2003; Martínez et al., 2004; Hilgert & Gil, 2006, 2007; Barboza et al., 2006; Goleniowsky et al., 2006; Estomba et al., 2006; Menseguez et al., 2007; Ladio & Lozada, 2009; Molares & Ladio, 2009, among others.
The first human populations that migrated to the Argentine territory twelve or thirteen thousand years ago were originally nomadic tribes of hunters and gatherers. By the year 1000 A.D., people all over the territory had already adopted some sort of agricultural and horticultural practices, using the fertile banks of some rivers after flood waters receded, irrigating some valleys in the arid west or burning patches in the forests of the humid northeast (Giberti, 2008).
In the 16th C, the Spanish conquerors of the geographical area comprising Argentina estimated the native population to be around half a million. These communities were characterized by diverse ethnic origin, social organization and cultural practices. Nowadays in Argentina the native population is represented by 18 ethnic groups (Zamudio, 2005) concentrated in the areas furthest away from the Pampas region, which occupies the centre of the country (Table 3). While some ethnic groups, as the yamanas and sel nam, have almost disappeared, others still make up important components of the population in the northwest (kolla), Chaco (chane, chiriguano, chorote, chulupi, mataco-wichi, mocovi, pilaga tapiete, toba), and Patagonia (tehuelches) (Zamudio, 2005).
TABLE 3 Aboriginal people of Argentina. Source: Zamudio (2005)
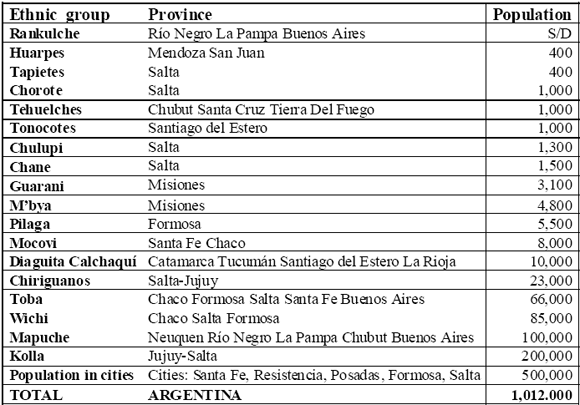
In contrast to other regions of America the cultures of these ethnic groups were not very well developed before the arrival of Europeans, with the exception of the kollas, of Incan origin. In Latin America, indigenous health systems today range from their traditional and isolated systems, increasingly threatened by deforestation, mining, and other activities, to systems strongly influenced by traditional western medicine. Between such extremes there is a gradient of intermediate systems. In many Indigenous communities, TM is still practised, with a link to allopathic medicine use. In addition to the use of traditional healers, known as Shamans (more formally Opygua, Pai, and other denominations), many families have their own knowledge and access to medicinal plants for use in emergencies (Montenegro & Stephens, 2006). The complete extinction of whole groups of Indigenous peoples, such as the Tonocotés, Lule-Vilela, Sanavirones, and Chana-Timbúes in Argentina, has almost certainly resulted in a loss of rich information on local medicinal plants and their ecosystems (Montenegro & Stephens, 2006).
In the Pampas, where the main economic activities of the country are concentrated, the native groups have lost their identity and have become totally absorbed by the national culture. This situation is reflected in the use of medicinal plants by the people. In rural areas it is still common to go to the 'curandero´. However, these days their use of medicinal plants is distorted, as they often prescribe 'herbs´ as cures, but they do so from a 'westernized´ perspective. The names used often correspond to traditional hispanic medicine, but the plants utilized usually have nothing to do with the original ones (Villamil, 2004).
In reference to NW Argentina Hilgert and Gil (2007) afirmed that the state of conservation of the traditional cultures of this region is variable and somewhat problematic but to a lesser or a greater extent all the peoples are related to an hegemonic culture. The Andean medical system, it is formed by a complex combination of herbal knowledge, magic elements and propitiatory rituals. The general cultural picture were described as a true tapestry of folk practices where the elements proper of the region and those that have been introduced by migrations both in pre-Columbian times as well as after the arrival of the Spanish are difficult to sort out.
Argentine medicinal plants are threatened by overharvesting, habit degradation, unsustainable cultivation practices, and lack of authoritative information. The most serious problem in relation to over-harvesting of the resource, and the consequent impact on its conservation, appears to be massive collection for commercialization in urban centres (Noher de Halac et al., 1985) rather than the effect that local use by indigenous groups would have on the plant populations. Understanding the fundamentals of the use and preservation of such a natural resource is thus a determining factor in the development of any research framework.
Among the immediate actions that are necessary to insure the survival of medicinal plant species, and the opportunity to use them sustainably, Villamil (2004) reported the following: (1) preparation of an up-to-date list of species used for medical purposes in the countries of the region; (2) survey of the state of their conservation using the categories of threat; (3) legislation concerning trade needs to be revised and its enforcement strengthened; development of methods of cultivation; (4) publicity campaign to instruct the public about this issue and (5) establishment of a national seed or germplasm bank.
The objectives of this work are as follows: 1) to introduce a general review of medicinal plants, 2) to report all scientific data available for us on ethnobotany, chemical composition, and biological activity of the native medicinal flora of Argentina, 3) to identify the plant parts used, and its application, 4) to consider the taxonomical richness of the native medicinal flora, 5) to determine endemism areas of the medicinal flora, and 6) to evaluate the impact of the native medicinal flora in bioprospecting.
Methodology
The Argentine medicinal flora is presented as a checklist where families, species, and infraspecific taxa are alphabetically arranged. The checklist only includes native species; naturalized or adventitious plants were excluded. The selection of the taxa from the literature was made following three criteria: 1. when the citation of a taxon specified therapeutic uses in Argentina; 2. when the citation of a taxon included the term 'medicinal' in describing the species uses in Argentina; 3. when the citation of a taxon reported specific medicinal applications, or the term 'medicinal' for Argentina's bordering countries.
The information was classified into four categories: Taxa, Ethnomedical information/ Distribution, Biological activities, and Chemical data.
In the Taxa column, details such as Family, Latin name, Vernacular name/s when known, Synonyms, and an Exsiccatum were specified. The nomenclature of the taxa was carefully controlled following different databases (Instituto de Botánica Darwinion, 2009), W3 Tropicos, Missouri Botanical Garden, St. Louis), and in some cases, according to the latest revision of the genera. The vernacular names were taken from the specimen labels, and according to De la Peña (1997), Orfila & D'Alfonso (1998), Novara (2003), Barbarán (2008), Peña-Chacarro et al. (2006), and Martínez (2008), among others. Synonyms are mentioned only when these names have been found in the literature indicating medicinal purposes.
In Ethnomedical information/distribution, medicinal uses are described according to the plant parts, and how the herbal is administered (infusion = hot H2O ext., decoction, juice, etc.). In case the literature does not indicate the organ used, then Part not specified is stated. The geographical distribution and status (native or endemic) of each taxon were taken from Zuloaga & Morrone (2008), and in some cases modified according to the latest revision of the genera. The acronomy for each Argentina province is: BAI (Buenos Aires), CAT (Catamarca), CHA (Chaco), CHU (Chubut), COR (Córdoba), COS (Corrientes), ERI (Entre Ríos), FOR (Formosa), JUJ (Jujuy), LPA (La Pampa), LRI (La Rioja), MEN (Mendoza), MIS (Misiones), NEU (Neuquén), RNE (Río Negro), SAL (Salta), SJU (San Juan), SLU (San Luis), SCR (Santa Cruz), SFE (Santa Fe), SDE (Santiago del Estero), TDF (Tierra del Fuego e islas del Atlántico Sur), TUC (Tucumán).
For chemical data and biological activity tested in vitro and in vivo, we conducted an electronic literature search of NAPRALERT (Natural Product Alert, University of Illinois, Chicago), MEDLINE, IBIDS (The International Bibliographic Information on Dietary Supplements), Science Direct, Pub Med, Bioline International Office Site, CNRS (Centre National de la Recherche Scientifique), Wiley InterScience, BioInfoBank Library, InformaWorld, and CAB Abstracts databases updated to March 2009, in addition to performing hand searches in other specific literature (Glasby, 1991; Harborne & Baxter, 1997; Hegnauer, 1973, 1986, 1989, 1990; Hegnauer & Hegnauer, 1994, Eich, 2008) among others. The keywords used for the electronic literature search for this review were scientific name and its synonym/s, medical uses, chemical studies, pharmacological activity, natural products. However, when such information was not provided, No biological test and No data were used respectively.
Results
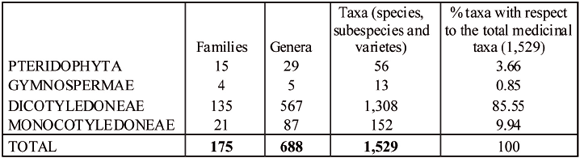
The recognized native medicinal flora of Argentina comprises some 1,529 taxa (species and ifraspecific taxa) grouped into 175 families and 688 genera of Vascular Plants (Pteridophyta, Gymnospermae and Angiospermae) (Table 4).
Table 4
Summary of the recognized medicinal vascular flora in Argentina
A detailed overview of all species registered, their specific and vernacular names, uses, pharmacological, and phytochemical data are given in Appendix I.
The vascular flora of Argentina includes ca. 10,937 taxa (Instituto de Botánica Darwinion, 2009). In this work, the taxa, used in folk medicine represent 14% of total flora. One thousand three hundred eight (85.555 % -from 1,529-) were Dictos, 152 (9.94%) Monocots, 56 (3.66%) Pterydophytes, and 13 (0.85%) Gymnosperms. Dictos and Monocots constitute together 95% of medicinal flora (Table 4).
Asteraceae is the family with the largest number of medicinal taxa (273 taxa, 17.85 %), followed by Fabaceae (113 / 7.39 %). Solanaceae (51 / 3.33 %), Euphorbiaceae (49 / 3.20 %), Verbenaceae (45 / 2.94 %), Poaceae (41 / 2.68 %), Apiaceae (35 / 2.28 %), Malvaceae (35 / 2.28 %), Rubiaceae (27 / 1.76 %) and Lamiaceae (26 / 1.70 %). It should be noted that 27 families constitute more than 75 % of total medicinal flora (Fig. 2).
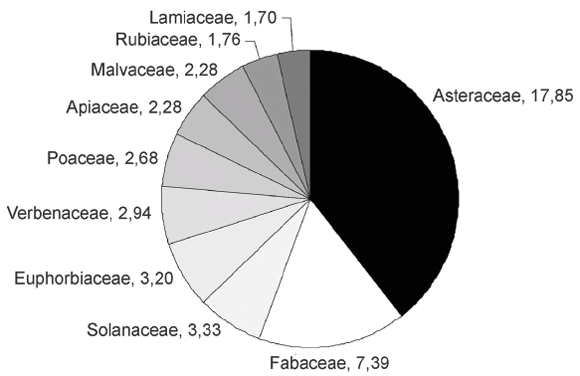
Fig. 2.- The most representative families with medicinal taxa in Argentina.
Twenty three different parts of the plant used to treat or alleviate diverse ailments or their effects were registered. Figure 3 shows the parts most frequently cited in the literature. As observed, almost all plant organs are used for medicinal purposes; the aerial parts (leaf, stem bark, etc.) being the most widely resorted to. It is important to point out that the part used is not always specified in the literature, as revealed by the 14% reported here. The leaves are the most used part, followed by stem, underground organs and finally flowers. The reproductive organs are less frequently mentioned. Flowers, fruits and seeds amount to 13 %, white roots and rhizomes make up 14%.
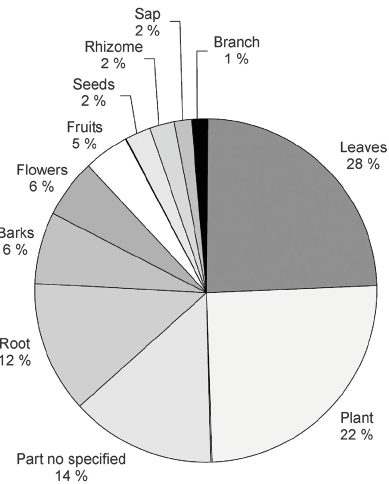
Fig. 3.- Most frequently used part in the native species of the Argentine medicinal flora.
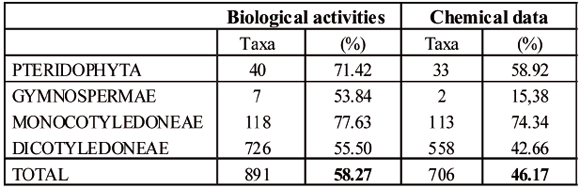
The chemical composition of more than 45 % of the taxa reported in this updated checklist hast not yet been analyzed. Monocootyledoneae (74.34 %) and Pteridophyta (58.92 %) are the groups least investigated so far. In the case of Dicotyledoneae, the percentage is also high especially considering that this group has the highest percentage in medicinal taxa. Lack of pharmacological knowledge is also more evident, since the biological activity of ca. 58 % of the medicinal flora has not been evaluated (Table 5).
Table 5
Group of plants whose biological activity and chemical data were not provided
Among the pharmacological activies, anti-inflammatory, antioxidant, and antimicrobial properties are the most tested up to now.
Figure 1 and Table 6 show the number of medicinal taxa, and the number of endemic medicinal taxa per province.
Table 6
Number of total taxa and medicinal taxa (number and percentage) per each Argentine province, including the number of endemic medicinal taxa

Salta (795 taxa), Jujuy (756), and Tucumán (666) in the northwestern region, and Corrientes (746), Misiones (596), Entre Ríos (564), and Chaco (619) in the northeastern region constitute the richest areas of medicinal plants in Argentina. A second group of importance has been found in the central area of the country, in the provinces of Córdoba (640 taxa), Buenos Aires (611), and Catamarca (598).
Considering the medicinal flora in relation to total flora per province, Santiago del Estero in the province with the hightest percentage of medicinal flora (46.06%), and Chaco (34.35). The lowest percentage observed is lower than 15 % (Tierra del Fuego) which highlights the importance attached to medicinal plants in all the geographic areas analyzed.
Among the medicinal flora found in Argentina, 7.52 % corresponds to endemics. The 116 endemic taxa (see Appendix III) belogn to 40 families, from which Asteraceae is the major one (36 medicinal endemic species) followed by Fabaceae (9 endemic taxa), Malvaceae (6), Verbenaceae (5), Apiaceae (4), Chenopodiaceae (4), Euphorbiaceae (4), and Polygalaceae (4). Genera with the highest numbers of endemic taxa are: Senecio (5), Sphaeralcea (5), Atriplex (4), Artemisia (3), Baccharis (3), Trichcline (3) and Lippia (3).
Average number of endemic taxa (average of provinces) is 28 (24 % with respect the total 116 endemics of Argentina) showing its importance at the scale of the country. The areas where the endemic species are mostly found follow a characteristic pattern: the largest amount of medicinal endemic species is located in the provinces of: Catamarca (48.69 % with respect total 115 medicinal endemics of Argentina), La Rioja (47.82 %), Mendoza (46.95%), San Luis (46.95%), Córdoba (45.21 %), and San Juan (43.47 %). When the relationships are referred with respect the total medicinal flora per province, Mendoza (14.28%), San Luis (13. 70%), and La Rioja (12.67%) are the main important. It appears that ecoregions of Monte and Chaco have the highest number of endemisms of medicinal plants.
According to the data shown, the provinces with greater diversity of medicinal flora match the type of species diversity and infraspecific taxa of the vascular flora reported by Zuloaga et al. (1999, 2008) and the pattern of Dicots diversity observed by Juárez et al. (2006): the Northeast and Northwest regions have the highest number of families, genera and species of medicinal plants. In the northwestern region, the richest area in medicinal species grows 2837 % of the endemics (Salta, Jujuy, and Tucumán, cfr. Table 6). Misiones, in turn, is the province with the highest rate of biodiversity (Zuloaga et al. 1999, 2008), but only with 2.60 % of medicinal endemic species.
It is interesting to point out that the medicinal endemics are not used profusely. Even more, 13 of these species are cited in the literature just as 'medicinal' without indicating a particular use or the organ used (e.g. Schinus johnstonii, Asclepias flava, Gochnatia glutinosa, Microliabum candidum, Cnidoscolus loasoides, etc.). The potential of these plants has not been evaluated in detail especially in its chemical compounds (No data: 68 species) and bioactivity (No biological test: 86 species).
In terms of conservation, 13 endemic species can be considered endangered since the only organ used in the majority of them is the root (Eryngium agavifolium, Hypochaeris pampasica, Senecio uspallatensis, Trichocline plicata, Trichocline sinuata, Berberis lilloana, Berberis grevilleana, Adesmia inflexa, Polygala stenophylla, and Valeriana ferax). Some species are very rare with a restricted distribution (Cyperus spectabilis var. jujuyensis, Chiliophyllum densifolium, Mutisia saltensis, Senecio pogonias, S. uspallatensis, Trichocline plicata, Senna kurtzii, Gentianella imberbis, G. parviflora, Sphaeralcea philippiana, Siphoneugena occidentalis) while others need priorities in conservation practices due to the progressive destruction of their habitats (e.g. Polylepis australis, Condalia microphylla, Lippia spp.).
Discussion
There is an estimated number of medicinal plants of 40,000-70,000 species in the world and still there is a lot of traditional knowledge that has not yet been really explored (Verpoorte, 2007). In Argentina, the composition of the native ethnopharmacopoeia has increased considerably specially with the contribution of detailed ethnobotany studies carried out in different aborigenous and rural communities where their healthcare systems continue to rely on their traditional plant-based medicines (Martínez Crovetto, 1964, 1965, 1968a, b, 1981; Arenas, 1981, 1983, 2000, 2003; Xifreda, 1992; Filipov, 1994, 1997; Del Vitto et al., 1997, Bocco et al., 1997; Pochettino et al., 1997; Amat & Yajía, 1998, Méndez, 1998; Pochettino & Martínez, 1998; Vignale, 1996, 1998; Gratti, 1998; Lahitte et al., 1998; Hilgert, 2001; Núñez & Cantero, 2000; Scarpa, 2002, 2004; Martínez, 2002, 2007a, b, 2008a, b; Kutschker et al., 2002; Roig, 2002; Martínez & Planchuelo, 2003; Martínez et al., 2004; Hilgert & Gil, 2006, 2007; Goleniowsky et al., 2006; Estomba et al., 2006; Menseguez et al., 2007; Ladio & Lozada, 2009; Molares & Ladio, 2009; Eyssartier et al., 2009). The 1,529 medicinal species registered in this review, which represent near the 15 % of the total Argentinean flora, demonstrate that we have a good source of promising plants that should be studied in detail. This value will be still greater if we also consider the introduced medicinal species.
Many of the industrially and commercially used pharmaceuticals derive from secondary metabolism produced by microbes, plants and animals. About 35,000 (some estimates increase the number to 70,000), out of 350,000 plant species known so far, serve medicinal purposes worldwide, and less than 0. 5 % have been chemically investigated (Shasany et al., 2007). The search for potentially active compounds has been the objective of several research groups and much progress has been done in the Argentinean medicinal flora in the last years. Despite of this, ca. a 46 % and a 58 % of the species remain unexplored in their chemical composition and biological activity, respectively, as it is shown in this paper.
This review showed that nearly 300 uses have been mentioned in literature although many of them are more or less similar. With the purpose to retain information as much as possible we only mention, from the Appendix I, 75 of them. General weakness, rheumatic pains, contusions and myalgia, kidney disorders, heart disorders, gastrointestinal upsets, neuralgias, coughs and colds, sexual disorders, are the most commonly reported indications to be treated with medicinal plants.
In Argentina, regional works had cited the gastro-intestinal illness as the most frequent problem whilst ailments connected with the nervous systems was the least frequent reported (Molares & Ladio, 2009). In this review, the pattern found was not so lineal. This highest frequency for the gastro-intestinal category has also been registered in numerous studies carried out in different human groups throughout the world (e.g., Milliken & Albert, 1997; Ankli et al., 1999; Schlage et al., 2000; Hilgert, 2001, Begossi et al., 2002, Scarpa, 2002). The high citing frequency of gastro-intestinal uses could be due to the kind of food ingested, foods not well preserved hygiene and also the easy diagnosis of these illness and effectiveness of different species to deal with them. During the last century, the traditional diet has undergone changes, resulting in an increase in the consumption of refined flour and meat, and a decrease in the consumption of wild and cultivated vegetables, leading to an unbalanced diet, lowing fibre content (Ferrari et al., 2004).
On the other hand, many of these communities have no supply of clean drinking water, their wells being situated close to latrines, or shared with farm animals, thus increasing the risk of ingesting pathogens.
The intensive harvesting of wild plants or their reproductive organs which evidence neglects in conservation practices or habitats, poses an increasing threat to the country's medicinal plants (Núñez & Cantero, 2000; Martínez et al., 2006). Medicinal plants are directly used from the wild. Their use is usually not fatal to individual plants, however in many cases like Hedeoma multiflorum, Minthostachys verticillata, Julocroton argenteus, Baccharis crispa, Trixis divaricata subsp. discolor, Passiflora caerulea, and Equisetum giganteum (Martínez et al., 2006), in Córdoba province, heavy or continued exploitation risks the regeneration of the natural sourced population. Perhaps the principal threat to medicinal species is nor over-harvesting but the destruction and conversion of their habitats to other purposes.
Many types of action can be taken in favour of the conservation and sustainable use of medicinal plants. Some of these are undertaken directly at the places where the plants are found, while others are less direct, such as some of those relating to commercial systems, ex situ conservation and bioprospecting. In the latter cases, actions taken will not lead to in situ conservation unless they feed back to improvements in the field. Probably the single most important role for medicinal plants in biological conservation is their 'use' to achieve conservation of natural habitats more generally. This stems from the special meanings that medicinal plants have to people, related to the major contributions that they make to many people's lives in terms of health support, financial income, cultural identity and livelihood security (Hamilton, 2004).
Land use has increased the rate of species extinction not only by replacing natural ecosystems, but also by changing the disturbance regime. In Argentina land cover changes associated with agriculture, grazing by domestic herbivores and deforestation have had an enormous impact on the structure and functioning of many ecosystems where medicinal plants are one of the main vegetation components, for example natural grasslands and native forest in intermountain valleys of Córdoba province (Cantero, pers. comm.). Other dramatic changes can be found for Tercero Arriba county, located in the same province (33°12'S, 64°W), in the Northern Espinal phytogeographic unit (Paruelo et al., 2001) where native woodlands were converted to introduce summer crops.
In order to set criteria to establish the conservation priorities of a species, based on its economic and social importance, and potential genetic erosion, a national program on medicinal germplasm conservation needs to be created. This national program should include: (1) ethnobotanical studies; (2) germplasm collection and characterization; and (3) ex situ and in situ conservation. Some criteria to define species priority conservation could be described as follows: (a) species with proven medicinal value, including those containing known active substance(s) or precursor(s) used in the chemical- pharmaceutical industry, or at least those demonstrating pre-clinical and toxicological results; (b) species with ethnopharmacological information widely used in TM, and which are threatened or vulnerable to extinction; (c) species with chemotaxonomic affinity which produce specific natural products.
Best representative families
The three best representative families with medicinal taxa in Argentina are Asteraceae, Fabaceae, and Solanaceae. Members of these families have been used by man for the prevention and relief of medical disorders since the dawn of civilization. Today, they continue to figure prominently in TM, especially in developing countries.
The economical and medicinal importance of the Asteraceae, one of the largest plant families in the world (ca. 23,000 species; cfr. Bremer, 1994), has been widely described (Heywood et al., 1977; Wagner, 1977; Emerenciano et al., 2007). Chemically, this family has been extremely studied and an enormous amount of information on its organic constituents is now available (Emerenciano et al., 2007). Among the great variety of chemical classes isolated from these plants, terpenoids, coumarins, flavonoids, polyacetylens, benzofurans, alkaloids, sesquiterpene lactones, essential oils, triterpenes, diterpenes, cyanogenic glycosides, and other minor constituents, can be cited (Hegnauer, 1977, 1989; Emerenciano, 2007). In our country, Asteraceae comprises ca. 1,490 species (indigenous and introduced) (Zuloaga et al., 1999), and 272 native taxa (ca. 18 %) have been reported with medicinal uses (this review). The genus Baccharis is one of the most important considering its enormous relevance regarding its medicinal, commercial, and biological applications (Bastos, 2008). Thirty six species (37.5 % ) of the 96 growing in Argentine (Giulano, 2000) have medicinal properties, and the majority of them has been studied phytochemically (ca. 70 %) or has at least one biological activity tested (52 %). Only four species (B. genistifolia, B. pingraea fo. angustissima, B. sculpta and B. vulneraria) lack of chemical data and biological test. Senecio and Eupatorium are also genera with high number of medicinal taxa (23 and 20 respectively), and coincidently with Baccharis, chemical and pharmacological information together is missing for a few members (cfr. Appendix I).
The second representative medicinal family is Fabaceae. The tremendous morphological diversity of this family due to the large number of species in the world (over 18,000), is also evidenced in the production of a large number of metabolites with considerable structural diversity (Waterman, 1994). The major groups of secondary metabolites are N-containing metabolites (true alkaloids, pseudoalkaloids, cyanogenic glycosides, non-protein amino acids, lectins), flavonoids, tannins, furocoumarins, anthraquinones, terpenes, etc. (Waterman 1994; Hegnauer & Hegnauer, 1994). The main importance of the Fabaceae is economical because of the seeds which are rich in high quality protein, providing man and animals with a highly nutritional food resource, besides of the value for its timber, dyes, resins, gums, insecticides, fibers, forage, fodder, ground cover, green manures, etc. (Allen & Allen, 1981). In terms of medicinal interest, more than 30 species had already been reported by Hieronymus (1882) in our territory, and nowadays 113 native medicinal taxa are registered (this review). Among them, ca. 42 % lacks of chemical information and ca. 65 % of pharmacological activity. However, much progress has been done in these aspects in some species, such as Bauhinia forficata var. pruinosa or Erythrina crista-galli, or the Acacia species.
In relation to Solanaceae (ca. 2,500 spp.), members of this family are valued in traditional and herbal medicine for treatment of a wide range of ailments on a global scale. Its contribution to medicine and drug therapy makes it one of the top-ranking families of drug-yielding plants (Roddick, 1991). Solanaceae provides a) drugs used in conventional chemotherapy, b) plants used in traditional/herbal medicine, and 3) plants used as a source of precursors of pharmaceutical steroids (Roddick, 1991). As occurs in Asteraceae and Fabaceae, this family is also diversified in the production of secondary chemical compounds, being the main ones: alkaloids (simple pyrrolidines, Nacylpyrrolidines, pyrrolidine-type nicotinoids, tropanes, calystegines, pyrrolizidines), tryptophan-derived alkaloids, phenylalanine-derived metabolites (phenylethylamine, phenylpropanoid acids, phenylethanoid alcohols, phenylmethanoids, capsaicinoids, hydroxycoumarins), hydroxycinnamate conjugates (hydroxycinnamoyl glucose esters and O-glucosides, chlorogenic acid, N-Acylramines), flavonoids, lignans, terpenoids (monoterpenoids, sesquiterpenoids, diterpenoids, triterpenoids, phytosterols, steroidal sapogenins/saponins, steroidal alkaloids), and fatty acids and their derivatives (Eich, 2008). The medicinal members of Solanaceae in Argentina comprise 52 taxa, and with the exception of the Solanum species, two species of Fabiana, and Grabowskia obtusa, the remaining ones have at least chemical or pharmacological information. The most promising species is Fabiana imbricata «palo pichi», in which many pharmacological studies have been focused (Alonso & Desmarchelier, 2006 and bibliography herein)
Among the remaining families that contribute with more than 1 % of medicinal taxa, Verbenaceae, Lamiaceae, Myrtaceae and Apiaceae are of central importance because of its aromatic members. The essential oils composition of several members of these families, with the exception of Apiaceae, have been reported to be effective as virucidal, antibacterial, antifungal, trypanocidal, acaricidal, nematicidal, fumigant and repellent against head lice, or to have analgesic, antioxidant, anti-diarrheic, spasmolytic, hepatotoxic, choleretic, antispasmodic, antiinflammatory, cytoprotective, anti-allergic, and lymphoproliferative activity (cfr. Appendix I). Medicinal and aromatic plants are a diverse group of plants with potential to contribute to economic development (Juliani et al., 2007). Zygadlo & Juliani (2003) and Juliani et al. (2007) reported the increasing commercial demand of herbal, medicinal and aromatic plants in Mid-Western Argentina. Many species included in Appendix I integrate the lists of species given by these authors.
Conclusions
- The recognized native medicinal flora of Argentina consists of 1,529 taxa. One hundred and fifteen taxa are endemics.
- Knowledge about the chemical composition and the biological activity of the medicinal flora needs to be further developed. More than 45 % of the species has not yet been chemically analyzed, while 58 % has not been tested pharmacologically on any subject considering the information available for us.
- Asteraceae is the richest family among medicinal taxa (272) and medicinal endemics (36).
- The northwestern and northeastern regions show the greatest diversity in terms of native medicinal flora.
- The provinces of Salta and Jujuy have the highest number of medicinal taxa in Argentina (795 and 756 taxa, respectively).
- The Monte ecoregion is the richest area in medicinal endemics. With respect the total medicinal endemic of the country, Catamarca has the highest percentage of endemic species (48.69 %) in its medicinal flora; at the same time Mendoza has the highest number of endemics (14.55%) respecting the medicinal flora per province. Formosa is one of the poorest areas in endemics medicinal plants (1.73%). Tierra del Fuego has not any endemic.
- Chemical constituents and biological activity are aspects scarcely explored in the medicinal flora of Argentina, probably due to a combination of lack of appropriate policies and laws to support the use of medicinal plants to fulfill healthcare needs, and low interest in their research among the scientific community. Therefore, due to its rich flora and empirical background which should not be ignored, pharmaceutical bioprospection is a promising ground.
- Relatively few native medicinal plants species are cultivated. The great majority is still provided by collection from the wild. This trend is likely to continue over the long term due to numerous factors: most medicinal plants are traded locally and regionally rather than internationally, the costs of domestication and cultivation are still high, and land in mainly used for cultivation of food crops. Wild collection practices are the unique secure valuable income for many rural households.
- For threatened and profitable medicinal plant species, like Minthostachys verticillata, Hedeoma multiflorum, Achyrocline satureoides, Passiflora caerulea, Acantholippia seriphioides, Lippia turbinata, Baccharis crispa, cultivation is a conservation option because the constant drain of material from their populations is much higher than the annual sustained yield. If the demand for these species can be met from cultivated sources the pressure on the wild populations will be relieved. In these cases, the need for strict conservation of remaining populations, improved security of germplasm ex situ and investment in selection and improvement programmes is extremely urgent. With respect to economic viability, as many other endangered species, as Gentianella parviflora, do not qualify for cultivation they will enter cultivation only with the help of public domestication programmes. For all other harvested medicinal species the priority conservation option is sustainable harvest from wild populations.
- Due to the richness and distribution of medicinal flora in Argentina, the importance of preserving the prevailing natural and seminatural ecosystems of our territory needs to be directly addressed. Overharvesting, land conversion (deforestation and clearing for agriculture, and urban development) poses a serious threat to many medicinal plants, especially given the small population density of some species or their restricted distribution. For these reasons, approaches to wild species collection that engage local, regional, and national collection enterprises and markets in the work of conservation and sustainable use of medicinal species are urgently needed. The ecoregions that deserve special attention due to the number of species collected are the following: Puna, Yungas Chaco, and Altos Andes, finally the Monte ecoregion deserves especial attention mainly due to its endemisms. A national program on medicinal germplasm conservation needs to be created.
Acknowledgements
The authors are especially grateful to colleagues who provided information or literature to complete the checklist. We would also like to thank the assistance of the technical staff of the Museo Botánico de Córdoba (UNC) and Facultad de Agronomía y Veterinaria (UNRC), and fruitful comments of the two anonymous reviewers on our manuscript. This work was supported by Secyt-UNC, Secyt-UNRC, Ministerio de Ciencia y Tecnología (Córdoba, Argentina), and CONICET.
APPENDIX I. Checklist of the native medicinal plants from Argentina
APPENDIX III Endemic species of the medicinal flora of Argentina (Family: Nº endemics)
Literature cited
1. Acosta Solís M. 1992. Vademecum de las plantas medicinales del Ecuador. Ediciones Abya-Yala, Quito. [ Links ]
2. Adams M., F. Gmunder & M. Hamburger. 2007. Plants traditionally used in age related brain disorders. A survey of ethnobotanical literature J. Ethnopharmacol. 113: 363-381. [ Links ]
3. Addae-Mensah I. 2000. Plant biodiversity, herbal medicine, intellectual property rights and industrially developing countries, en H. Lauer (ed.). Ghana: Changing values/changing technologies, pp. 165-182. Council for Research in Values and Philosophy, Washington, DC. [ Links ]
4. Aguilar-Støen M. & S. R. Moe. 2007. Medicinal plant conservation and management: distribution of wild and cultivated species in eight countries. Biodiversity & Conservation 16: 1973-1981. [ Links ]
5. Albuquerque U. 2006. Re-examining hypotheses concerning the use and knowledge of medicinal plants: a study in the Caatinga vegetation of NE Brazil. J. Ethnobiol. Ethnomed. 2: 1-10. [ Links ]
6. Allen O. N. & E. K. Allen. 1981. The Leguminosae. A source book of characteristic, uses and nodulation. The University of Wisconsin Press, Madison. [ Links ]
7. Alonso J. & C. Desmarchelier. 2006. Plantas medicinales autóctonas de la Argentina. Bases científicas para su aplicación en atención primaria de la salud. Ediciones Fitociencia, Capital Federal. [ Links ]
8. Alves R. & I. M. L. Rosa. 2007. Biodiversity, TM and public health: where do they meet? J. Ethnobiol. Ethnomed. 3: 1-9. [ Links ]
9. Amat A. G. & M. Yajía. 1998. Las plantas vasculares utilizadas en la fitoterapia popular de la provincia de Misiones, en G. Amat (coord.), Farmacobotánica y Farmacognosia en Argentina 1980-1998, pp. 119-152. Ediciones Científicas Americanas, La Plata. [ Links ]
10. Amorín J. L. & R. A. Rossow. 1989-1992. Guía taxonómica con plantas de interés farmacéutico. Dominguenzia 7 (1): 31-38; 8(1): 28-33; 9 (1) 54-62; 10 (1): 35-40. [ Links ]
11. Amorín J. L. 1980-1981. Guía taxonómica con plantas de interés farmacéutico. Rev. INFYB 3 (5-6): 7-28; 4 (78): 95-112; 4 (9): 193-207; 4 (10):245-259. [ Links ]
12. Ankli A., O. Sticher & M. Heinrich. 1999. Medical ethnobotany of the Yucatec Maya: Healers' consensus as a quantitative criterion. Econ. Bot. 53: 144-160. [ Links ]
13. Arbo M. M. & S. G. Tressens (eds). 2002. Flora del Iberá. EUDENE, Corrientes. [ Links ]
14. Arenas P. 1981. Etnobotánica Lengua-Maskoy. FECIC, Buenos Aires. [ Links ]
15. Arenas P. 1983. Nombres y usos de las plantas por los indígenas Maká del Chaco Boreal. Parodiana 2: 131-229. [ Links ]
16. Arenas P. 2000. Farmacopea y curación de enfermedades entre algunas etnias del Gran Chaco, en A. G. Amat (coord.). Farmacobotánica y Farmacognosia en Argentina 1980-1998, pp. 87-118. Ediciones Culturales Argentina (ECA), La Plata. [ Links ]
17. Arenas P. 2003. Etnografía y alimentación entre los tobañachilamole#ek y wichí-lhuku'tas del Chaco Central (Argentina). Editorial Latín Gráfica S.R.L, Buenos Aires. [ Links ]
18. Arias T. D. 1999. Glosario de Medicamentos: desarrollo, evaluación y uso. Organización Panamericana de la Salud. OMS, Washington D. C. [ Links ]
19. Arnason J. T., P. M. Catling, E. Small, P.T. Dang & J. D. H. Lambert. 2005. Biodiversity & Health: Focusing Research to Policy. NRC Research Press, Ottawa, Canadá [ Links ].
20. Arnold T. H., C. A. Prentice, L. C. Hawker, E. E. Snyman, M. Tomalin, N. R. Crouch & C. Pottas-Bircher. 2000. Medicinal and magical plants of Southern Africa: an annotated checklist. Strelitzia II. National Botanical Institute, South Africa. [ Links ]
21. Au D.T., J. Wu, Z. Jiang, H. Chen, G. Lu & Z. Zhao. 2008. Ethnobotanical study of medicinal plants used by Hakka in Guangdong, China. J. Ethnopharmacol. 117: 41-50. [ Links ]
22. Balick M. J. & P. A. Cox. 1996. Plants, people and culture: the science of ethnobotany. The Scientific American Library, New York. [ Links ]
23. Balick M. J., E. Elisabetsky & S. A. Laird. 1996. Medicinal resources of the Tropical Forest: Biodiversity and its importance to Human Health. Columbia University Press, New York. [ Links ]
24. Bannerman R. H., J. Burton & Ch. Wen-Chieh. 1983. TM and health care coverage: a reader for health administrators and practitioners. World Health Organization 1983: 318-27. Geneva, Switzerland. [ Links ]
25. Barbarán F. R. 2008. Medicinal Plants of the Argentinian Puna: A common property resource and an opportunity for local people. Conference at «Governing Shared Resources: Connecting Local Experience to Global Challenges» 12th Biennial Conference of the International Association for the Study of Commons, Cheltenham, England. [ Links ]
26. Barboza G. E., J. J. Cantero, C. O. Nuñez & L. Ariza Espinar (eds.). 2006. Flora Medicinal de la Provincia de Córdoba (Argentina). Pteridófitas y Antófitas silvestres o naturalizadas. Museo Botánico, Córdoba. [ Links ]
27. Barboza, G. E., A. T. Hunziker, G. Bernardello, A. A. Cocucci, C. Carrizo García, V. Fuentes, M. Dillon, V. Bittrich, M. T. Cosa, R. Subils, A. Romanutti, S. Arroyo & A. M. Anton. Solanaceae. A ser editado por K. Kubitzki, The Families and Genera of Vascular Plants (Alemania). Submitted. [ Links ]
28. Bastos J. K. 2008. Chemistry of Baccharis genus. Conferences Abstracts XX Simpósio de Plantas Medicinais do Brasil & X International Congress of Ethnopharmacology: 30. São Paulo, Brasil. [ Links ]
29. Basualdo I., E. Zardini & M. Ortiz. 1991. Medicinal plants of Paraguay: Underground organs. Econ. Bot. 45: 86-96. [ Links ]
30. Begossi A., N. Hanazaki & J. Y. Tamashiro. 2002. Medicinal plants in the Atlantic Forest (Brazil): knowledge, use, and conservation. Human Ecol. 30: 281-299. [ Links ]
31. Bisset N. (ed.). 1994. Herbal drugs and phytopharmaceuticals. A handbook for practice on a scientific basis. Ed. Medpharm. Sc. Publishers, Sttutgarts-CRC Press, Boca Raton. [ Links ]
32. Blacpma. 2006. Primer Simposio Internacional de Plantas Medicinales y Aromáticas: materias primas, puntoscríticos y rol de las universidades, Los Ángeles, Chile, Vol. 5 (6). [ Links ]
33. Blacpma. 2007. IX Simposio Argentino y XII Simposio Latinoamericano de Farmacobotánica, Tucumán (Argentina). Vol. 6 (5). [ Links ]
34. Bocco M. E., N. Vischi & N. Montani. 1997. Relevamiento de las plantas medicinales espontáneas del departamento Río Cuarto (Córdoba, Argentina). Parodiana 10: 11-18. [ Links ]
35. Bodeker G. 1994. Traditional health knowledge and public policy. Nature & Resources 30: 5-16. [ Links ]
36. Bramwell D. 2003. On the size of the world's threatened flora. Plant Talk 32: 4-5 [ Links ]
37. Bremer K. 1994. Asteraceae. Cladistics & Classification. Timber Press, Inc., Oregon. USA. [ Links ]
38. Briskin D. 2000. Medicinal plants and phytomedicines. Linking plant biochemistry and physiology to human health. Pl. Physiol. 124: 507-514. [ Links ]
39. Brouwer N., Q. Liu, D. Harrington, J. Kohen, S. Vemulpad, J. Jamie, M. Randall & D. Randall. 2005. An ethnopharmacological study of medicinal plants in New South Wales. Molecules 10: 1252-1262. [ Links ]
40. Bussmann R. & D. Sharon. 2006. Traditional medicinal plant use in Northern Peru: tracking two thousand years of healing culture. J. Ethnobiol. Ethnomed. 2: 47 [ Links ]
41. Caldecott J. O., M. D Jenkins, T. H. Johnson & B. Groombridge. 1996. Priorities for conserving global species richness and endemism. Biodiversity & Conservation 5: 699-727. [ Links ]
42. Calixto J. 2005. Twenty-five years of research on medicinal plants in Latin America. A personal view. J. Ethnopharmacol. 100: 131-134. [ Links ]
43. Chaves R. 2001. Medicinal Plants, Folk Medicine, Ethnomedicine, History and Pharmacology. Universidad para la Paz, UNESCO, San José, Costa Rica. [ Links ]
44. Coe F. 2008. Ethnomedicine of the rama of southeastern Nicaragua. J. Ethnobiol. 28: 1-38. [ Links ]
45. Comer M. & E. Debus. 1996. A partnership: Biotechnology, biopharmaceuticals and biodiversity, en F. di Castri & T. Younnes (eds.). Biodiversity. Science and Development, pp. 488-499. CABI Publ, Oxford, UK. [ Links ]
46. Cordell G. & M. Colvard. 2005. Some thoughts on the future of ethnopharmacology. J. Ethnopharmacol. 100: 5-14. [ Links ]
47. Cordell G. 2000. Biodiversity and drug discovery - a symbiotic relationship. Phytochemistry 55: 463-480. [ Links ]
48. Cotton C. M. 1997. Ethnobotany. Principles and Applications. Wiley. [ Links ]
49. Cox P. A. & M. J. Balick. 1994. The ethnobotanical approach to drug discovery. Scientific American 270: 82-87. [ Links ]
50. Croteau R., T. M. Kutchan & N. G. Lewis. 2000. Natural products (secondary metabolites), en B. Buchanan, W. Gruissem & R. Jones (eds.). Biochemistry and Molecular Biology of Plants, pp. 1250-1318. American Society of Plant Physiologists, Rockville, MD, USA. [ Links ]
51. Cunningham A. B. 1994. Integrating local plant resources and habitat management. Biodiversity & Conservation 3: 104-115. [ Links ]
52. Daniel M. 2006. Medicinal Plants: Chemistry and Properties. Science Publishers, Texas, USA. [ Links ]
53. Davis S. D., V. H. Heywood, O. Herrera-MacBryde, J. Villa-Lobos & A. Hamilton (eds.). 1997. Centres of Plant Diversity: A Guide and Strategy for Their Conservation. Vol. 3. The Americas. IUCN Publications Unit, Cambridge, England. http://www.nmnh.si.edu/botany/projects/cpd/. [ Links ]
54. De la Peña M. R. 1997. Catálogo de nombres vulgares de la Flora Argentina (lista preliminar). Universidad Nacional del Litoral, Santa Fe. [ Links ]
55. Del Vitto L. A., E, M. Petenatti & M. E. Petenatti. 1997. Recursos herbolarios de San Luis (República Argentina). Primera parte: Plantas nativas. Multequina 6: 49-66. [ Links ]
56. Delucchi G. 2006. Las especies vegetales amenazadas de la Provincia de Buenos Aires: Una actualización. APRONA Bol. Cient. 39: 19-31. [ Links ]
57. Dev S. 1999. Ancient-modern concordance in Ayurvedic plants: some examples. Environ. Health Perspectives 107: 783-789. [ Links ]
58. Dewick P. M. 2009. Medicinal plants products: a biosynthetic approach. John Wiley and Sons, Baffins Lane, Chichester, UK. [ Links ]
59. Dhillion S. S. & H. Svarstad, C. Amundsen & H. C. Bugge. 2002. Bioprospecting effects on development and environment. AMBIO 31: 491-493. [ Links ]
60. Dhillion S. S. & L. Ampornpan. 2000. Bioprospecting and phytomedicines in Thailand: conservation, benefit sharing and regulations, en H. Svarstad & S. S. Dhillion (eds.). Responding to Bioprospecting: from biodiversity in the south to medicines in the north. Spartacus Forlag AS, Oslo. [ Links ]
61. Díaz S., J. Fargione, F. Stuart Chapin & D. Tilman. 2006. Biodiversity loss threatens human well-being. PLoS Biol 4: e277. DOI: 10.1371/journal. pbio.0040277 [ Links ]
62. Domínguez J. 1928. Contribuciones a la materia médica Argentina. J. Peuser, Buenos Aires. [ Links ]
63. Eich E. 2008. Solanaceae and Convolvulaceae: Secondary metabolites. Springer-Verlag, Berlin Heidelberg. [ Links ]
64. Emerenciano V. P., M. J. P. Ferreira, M. T. Scotti, M. V. Correia, S. A. Alvarenga & G. V. Rodrigues. 2007. Use of backpropagation artificial neural networks to predict the occurrences of chemical classes in Asteraceae. XXVII Annual Meeting on Micromolecular Evolution, Systematics and Ecology Reflections on the Current Status of Chemosystematics. RPS-2. São Paulo, Brasil. [ Links ]
65. Estomba D., A. Ladio & M. Lozada. 2006. Medicinal wild plant knowledge and gathering patterns in a Mapuche community from North-western Patagonia. J. Ethnopharmacol. 103: 109-119. [ Links ]
66. Etkin N. L. 2006. Edible Medicines: An Ethnopharmacology of Food. University of Arizona Press. Tucson, Arizona, USA. [ Links ]
67. Eyssartier C., A. H. Ladio & M. Lozada. 2009. Uso de plantas medicinales cultivadas en una comunidad semi-rural de la estepa patagónica. Blacpma 8 (2): 77-85. [ Links ]
68. Farnsworth N. R. & D. D. Soejarto. 1991. Global importance of medicinal plants, en O. Akerele, V. Heywood & H. Synge (eds.). Conservation of Medicinal Plants, pp. 25-51. Cambridge University Press, Cambridge, UK. [ Links ]
69. Ferrari M. A., F. Morazán & L. V. Pinotti. 2004. Patrón alimentario de una comunidad aborigen de la Patagonia Argentina. Rev. Chil. Nutrición 31: 110-117. [ Links ]
70. Filipov A. 1994. Medicinal plants of the Pilagá of Central Chaco. J. Ethnopharm. 44: 181-193. [ Links ]
71. Filipov A. 1997. La farmacopea natural en los sistemas terapéuticos de los indígenas Pilagá. Parodiana 10: 53-74. [ Links ]
72. Fonnegra Gómez R, & S. L. Jiménez Ramírez. 2007. Plantas medicinales aprobadas en Colombia, 2da. Ed., Editorial Universidad de Antioquia, Antioquia. [ Links ]
73. Fowler M. W. 2006 Plants, medicines and man. J. Sci. Food & Agric. 86: 1797-1804 [ Links ]
74. Freire S. 1998. Especies medicinales de la provincia biogeográfica pampeana, en G. Amat (coord.). Farmacobotánica y Farmacognosia en Argentina 1980-1998, pp. 153-166. Ediciones Científicas Americanas, La Plata. [ Links ]
75. Gaikwad J., V. Khanna, S. Vemulpad, J. Jamie, J. Kohen & S. Ranganathan. 2008. CMKb: a web-based prototype for integrating Australian Aboriginal customary medicinal plant knowledge. BMC Bioinformatics 9 (Suppl 12): S25. doi:10.1186/14712105-9-S12-S25 [ Links ]
76. Garrido G. 2008. Gran diccionario de las plantas medicinales: propiedades curativas. Libro Hobby Club, Madrid. [ Links ]
77. Giberti G. 2008. Potential medicinal flora of Argentina. http://www.ics.trieste.it/Documents/Downloads/df1799.pdf. [ Links ]
78. Giuliano D. A. 2000. Asteraceae. Tribu III. Astereae, parte A. Subtribu Baccharinae. Flora Fanerogámica Argentina 66: 1-73. PROFLORA-CONICET [ Links ]
79. Glasby J. 1991. Dictionary of plants. Containing secondary metabolites. Ed. Taylor & Francis, London. [ Links ]
80. Goleniowski M. E., G. A. Bongiovanni, L. Palacio, C. O. Núñez & J. J. Cantero. 2006. Medicinal plants from the «Sierra de Comechingones», Argentina. J. Ethnopharmacol. 107: 324-341. [ Links ]
81. González Torres D. M. 2005. Catálogo de Plantas Medicinales (y alimenticias y útiles) usadas en el Paraguay. Servilibro, Asunción. [ Links ]
82. Gratti A. 1998. El aporte de la etnobotánica al estudio de la Farmacobotánica: la región patagónica, en G. Amat (coord.). Farmacobotánica y Farmacognosia en Argentina 1980-1998, pp. 167-184. Ediciones Científicas Americanas, La Plata. [ Links ]
83. Grifo F., D. Newman, A. S. Fairfield, B. Bhattacharya & J. T. Grupenhoff. 1997. The origins of prescription drugs, en F. Grifo & J. Rosenthal (eds.). Biodiversity and human health, pp. 131-163. Island Press, Washington, D.C. [ Links ]
84. Grünwald J. & K. Büttel. 1996. The European phytotherapeutics market. Drugs Made in Germany 39: 6-11. [ Links ]
85. Gupta M. P. (ed.). 1995. 270 Plantas Medicinales Iberoamericanas. CYTED-SECAB, Bogotá [ Links ].
86. Gurib-Fakim A. & T. Brendler. 2004. Medicinal and Aromatic Plants of Indian Ocean Islands. Medpharm GmbH, Scientific Publishers, Stuttgart, Germany. [ Links ]
87. Gurib-Fakim A. 2006. Traditions of yesterday and drugs of tomorrow. Molecular Aspects of Medicine. Special Edition. Elsevier Publications, UK. [ Links ]
88. Halberstein R. 2005. Medicinal Plants: historical and cross-cultural usage patterns. Ann. Epidemiol. 15: 686-699. [ Links ]
89. Hall P. & K. Bawa. 1993. Methods to assess the impact of extraction of non-timber tropical forest products on plant populations. Econ. Bot. 47: 234-247. [ Links ]
90. Hamayun M., S. Afzal Khan, E. Young Sohn & I. Lee. 2006. Folk medicinal knowledge and conservation status of some economically valued medicinal plants of District Swat, Pakistan. Lyonia 11: 101-113. [ Links ]
91. Hamilton A. C. 2004. Medicinal plants, conservation and livelihoods. Biodiversity & Conservation 13: 14771517. [ Links ]
92. Harborne J. B. & H. Baxter. 1997. Dictionary of plant toxins. Wiley & Sons, England. [ Links ]
93. He S. A. & Y. Gu. 1997. The challenge for the 21st Century for Chinese botanic gardens, en D. H. Touchell & K. W. Dixon (eds.). Conservation into the 21st Century, pp. 21-27. Kings Park and Botanic Garden, Perth. [ Links ]
94. Hegnauer R. 1973. Chemotaxonomie der Pflanzen. Band 6. Birkhäuser Verlag Basel, Germany. [ Links ]
95. Hegnauer R. 1977. The chemistry of the Compositae, en V. H. Heywood, J. B. Harborne & B. L. Turner (eds.). The Biology and Chemistry of the Compositae I, pp. 284-335. Academic Press INC, London. [ Links ]
96. Hegnauer R. 1986. Chemotaxonomie der Pflanzen. Band Birkhäuser Verlag Basel, Germany. [ Links ]
97. Hegnauer R. 1989. Chemotaxonomie der Pflanzen. Band Birkhäuser Verlag Basel, Germany. [ Links ]
98. Hegnauer R. 1990. Chemotaxonomie der Pflanzen. Band 9. Birkhäuser Verlag Basel, Germany. [ Links ]
99. Hegnauer R. & M. Hegnauer. 1994. Chemotaxonomie der Pflanzen. Band 11a. Birkhäuser Verlag Basel, Germany. [ Links ]
100. Heinrich M. & S. Gibbons. 2001. Ethnopharmacology in drug discovery: an analysis of its role and potential contributions. J. Pharm. & Pharmacol. 53: 425-432. [ Links ]
101. Herrera Vásquez S. & E. Rodríguez Yunta. 2004. Ethnoknowledge in Latin America. Appropriation of genetic resources and bioethics. Acta Bioeth. 10: 1-10. [ Links ]
102. Hewson M. G. 1998. Traditional healers in Southern Africa. Ann. Internal Med. 128:1029-1034. [ Links ]
103. Heywood V. H., J. B. Harborne & B. L. Turner. 1977. An overture to the Compositae, en V. H. Heywood, J. B. Harborne & B. L. Turner (eds.). The Biology and Chemistry of the Compositae I, pp. 1-20. Academic Press INC, London. [ Links ]
104. Hieronymus J. 1882. Plantae diaphoricae florae argentinae. Ed. Kraft, Buenos Aires. [ Links ]
105. Hilgert N. 2001. Plants used in home medicine in the Zenta River basin, Northwest Argentina. J. Ethnopharm. 76: 11-34. [ Links ]
106. Hilgert N. I. & G. E. Gil. 2006. Medicinal plants of the Argentine Yungas plants of the Las Yungas biosphere reserve, Northwest of Argentina, used in health care. Biodiversity & Conservation 15: 2565-2594. [ Links ]
107. Hilgert N. I. & G. E. Gil. 2007. Reproductive medicine in northwest Argentina: traditional and institutional systems. J. Ethnobiol. Ethnomed. 3: 1-13. [ Links ]
108. Instituto de Botánica Darwinion (on line). 2009. Flora del Conosur. Catálogo de las Plantas Vasculares. http://www.darwin.edu.ar/Proyectos/FloraArgentina/FA.asp. > [acceded 2009/20/03 ]. [ Links ]
109. IUCN. 1993. WHO/IUCN/WWF Guidelines on the Conservation of Medicinal Plants. 38p. IUCN, Gland, Switzerland. [ Links ]
110. Jain S. K. 2008. Human aspects of plant diversity. Econ. Bot. 54: 459-470. [ Links ]
111. Juárez A., P. Ortega-Baes, S. Suhring, W. Martin & G. Galíndez. 2007. Spatial patterns of dicot diversity in Argentina. Biodiversity & Conservation 16: 1669-1677. [ Links ]
112. Juliani H. R., F. N. Biurrun, A. Koroch, A. De Carli & J. A. Zygadlo. 2007. The production of native and exotic herbs, medicinal and aromatic plants in Argentina, en J. Janick & A. Whipkey (eds.). Issues in new crops and new uses, pp. 316-321. ASHS Press, Alexandria, VA. [ Links ]
113. Kaufman P. B., L. J. Cseke, S. Warber, J. A. Duke & H. L. Brielmann. 1999. Natural Products from Plants. CRC Press, Boca Raton, FL. [ Links ]
114. Khare C. 2007. Indian medicinal plants: an illustrated dictionary. Springer Verlag Inc., Berlin-Heidelberg. [ Links ]
115. Kinghorn A. D. 2002. Overview, en D. A. Kinghorn (ed.). Stevia: the Genus Stevia, Medicinal and Aromatic Plants-Industrial Profiles 19, pp. 1-17, Taylor & Francis, London and New York. [ Links ]
116. Kultur S. 2007. Medicinal plants used in Kýrklareli Province (Turkey). J. Ethnopharmacol. 111: 341-364. [ Links ]
117. Kumara B., M. Vijayakumar, R. Govindarajan & P. Pushpangadan. 2007. Ethnopharmacological approaches to wound healing-Exploring medicinal plants of India. J. Ethnopharmacol. 114:103-113. [ Links ]
118. Kutscher A., H. Menoyo & V. Hechem. 2002. Plantas medicinales de uso popular en comunidades del oeste de Chubut. E.E.A. Universidad Nacional de la Patagonia y GTZ, Esquel. [ Links ]
119. Ladio A. H. & M. Lozada. 2009. Human ecology, ethnobotany and tradicional practices in rural populations inhabiting the Monte region: Resilience and ecological knowledge. J. Arid Environ. 73: 222-227. [ Links ]
120. Lahite H. B., J. A. Hurrell, L. Jankowski, P. Haloua & K. Mehltreter. 1998. Plantas medicinales rioplatenses. Ed. L.O.L.A., Buenos Aires [ Links ]
121. Lahitte H. B. & J. A. Hurrell. 1996. Las plantas de la medicina popular de la isla Martín García (Nativas y Naturalizadas). CIC, Buenos Aires. [ Links ]
122. Laird S. A. & A. R. Pierce. 2002. Promoting sustainable and ethical botanicals: strategies to improve commercial raw material sourcing: results from the sustainable botanicals pilot project industry surveys, case studies and standards collection. Rainforest Alliance, New York. [http://www.rainforestalliance.org/news/2002/botanicals-strategies.pdf] [ Links ]
123. Lambert J., J. Srivastava & N. Vietmeyer. 1997. Medicinal Plants. Rescuing a Global Heritage. World Bank Technical Paper 355. Washington, DC. [ Links ]
124. Leaman D. J. & S. Salvador. 2005. An international standard for the sustainable wild collection of medicinal and aromatic plants (ISSC-MAP): principles, criteria, indicators, and means of verification. Draft 2, April 2005. Steering Group for the Development of Practice Standards and Performance Criteria for the Sustainable Wild Collection of Medicinal and Aromatic Plants. [ Links ]
125. Libman A., S. Bouamanivong, B. Southavong, K. Sydara & D. Soejarto. 2006. Medicinal plants: An important asset to health care in a region of Central Laos. J. Ethnopharmacol. 106: 303-311. [ Links ]
126. Lulekal E., E. Kelbessa, T. Bekele & H. Yineger. 2008. An ethnobotanical study of medicinal plants in Mana Angetu District, southeastern Ethiopia. J. Ethnobiol. Ethnomed. 4: 1-10. [ Links ]
127. Manandhar N. P. 1994. An ethnobotanical survey of herbal drugs of Kaski District, Nepal. Fitoterapia 65: 7-13. [ Links ]
128. Manandhar N. P. 2002. Plants and People of Nepal. Timber Press, Inc., Portland, U.S.A. [ Links ]
129. Martínez Crovetto R. 1964. Estudios etnobotánicos I. Nombres de plantas y su utilidad, según los indios tobas del E del Chaco. Bonplandia 1: 279-333. [ Links ]
130. Martínez Crovetto R. 1965. Estudios etnobotánicos II. Nombres de plantas y su utilidad, según los indios vilelas. Bonplandia 2: 1-23. [ Links ]
131. Martínez Crovetto R. 1968a. Estudios etonobotánicos III. Nombres de plantas y su utilidad, según los indios araucano-pampas del oeste de Buenos Aires (Argentina). Etnobiología 12: 1-14. [ Links ]
132. Martínez Crovetto R. 1968b. Estudios etonobotánicos IV. Nombres de plantas y su utilidad, según los indios onas de Tierra del Fuego. Etnobiología 3: 1-20. [ Links ]
133. Martínez Crovetto R. 1981. Las plantas utilizadas en medicina popular en el noroeste de Corrientes (Argentina). Miscelánea Fund. Miguel Lillo 69: 1-139. [ Links ]
134. Martínez G. J. & A. M. Planchuelo. 2003. La medicina tradicional de los criollos campesinos de Paravachasca y Calamuchita, Córdoba (Argentina). Scripta Ethnologica 25: 83-116. [ Links ]
135. Martínez G. J. 2002. Conocimiento de la flora de interés etnobotánico entre estudiantes del Valle de Paravachasca, Córdoba (Argentina). Parodiana 12: 35-62. [ Links ]
136. Martínez G. J. 2007a. La farmacopea natural en la salud materno-infantil de los tobas del Río Bermejito. Kurtziana 33: 39-64. [ Links ]
137. Martínez G. J. 2007b. Medicinal plants used by the Criollos of Calamuchita to treat blood, cardiovascular, and neuroendocrinous diseases. J. Herbs, Spices & Medicinal Plants 13: 55-82. [ Links ]
138. Martínez G. J. 2008a. Farmacopea natural y tratamiento de afecciones de la piel en la medicina tradicional de los campesinos de las sierras de Córdoba (República Argentina). Dominguezia 24: 27-46. [ Links ]
139. Martínez G. J. 2008b. Traditional practices, beliefs and uses of medicinal plant in relation to the maternal- baby health of the Criollo woman in Central Argentina. Midwifery 24: 490-502. [ Links ]
140. Martínez G. J., A. M. Planchuelo, E. Fuentes & M. Ojeda. 2006. A numeric index to establish conservation priorities for medicinal plants in the Paravachasca Valley, Córdoba, Argentina. Biodiversity & Conservation 15: 2457-2475. [ Links ]
141. Martínez M. R., M. L. Pochettino & A. R. Cortella. 2004. Environment and illness in the Calchaquí Valley (Salta, Argentina): phytotherapy for osteo-articular and cardio-circulatory diseases. J. Ethnopharmacol. 95: 317-327. [ Links ]
142. Marzocca A. 1997. Vademécum de malezas medicinales de la Argentina (nativas y exóticas). Ed. Orientación gráfica, Buenos Aires. [ Links ]
143. Massardo F. & R. Rozzi. 1996. Usos medicinales de la flora nativa chilena. Ambiente & Desarrollo 12: 76-81. [ Links ]
144. McChesney J., S. Venkataraman & J. Henri. 2007. Plant natural products: Back to the future or into extinction? Phytochemistry 68: 2015-2022. [ Links ]
145. McClatchey W. 2005. Medicinal bioprospecting and ethnobotany research. Ethnobot. Res. & Applications 3: 189-190. [ Links ]
146. McGaw L., A. Jäger, O. Grace, C. Fennel & J. van Staden. 2005. Medicinal plants, en A. van Niekerk (ed.). Ethics in Agriculture. An African Perspective, pp. 67-83. Springer, The Netherlands. [ Links ]
147. Mendelsohn R. & M. J. Balick. 1995. The value of undiscovered pharmaceuticals in tropical forests. Econ. Bot. 49: 223-228. [ Links ]
148. Méndez E. 1998. Plantas medicinales en las comunidades vegetales de Cacheuta, Mendoza Argentina. Rev. Estudios Regionales 19: 185-196. [ Links ]
149. Menseguez P., L. Galetto & A. M. Anton. 2007. El uso de plantas medicinales en la población campesina de El Puesto (Córdoba, Argentina). Kurtziana 33: 89102 [ Links ]
150. Mereles M. F. 2009. La diversidad vegetal en el Paraguay, en A. Pin & G. Céspedes (eds.). 2009. Plantas Medicinales del Jardín Botánico de Asunción, pp. 347-354. Asociación Etnobotánica Paraguaya, Asunción. [ Links ]
151. Mertz O., H. Munk Ravnborg, G. Lövei, I. Nielsen & C. Konijnendijk. 2007. Ecosystem services and biodiversity in developing countries. Biodiversity & Conservation 16: 2729-2737. [ Links ]
152. Miller J. S. & S. J. Brewer. 1992. The discovery of medicines and forest conservation, en R. P. & J. E. Adams (eds.). Conservation of plant genes: DNA banking and in vitro biotechnology, pp. 119-134. Academic Press, San Diego. [ Links ]
153. Milliken W.& B. Albert. 1997. The use of medicinal plants by the Yanomami Indians of Brazil. Econ. Bot. 51: 264-278. [ Links ]
154. Moerman D. E. 1998. Native North American food and medicinal plants: epistemological considerations, en H. D.V. Prendergast, N. L. Etkin, D. R. Harris & P. J. Houghton (eds.). Plants for food and medicine, pp. 69-74. Proc. Joint Conference of the Society for Economic Botany and the International Society for Ethnopharmacology, Royal Botanic Gardens, Kew. [ Links ]
155. Mohagheghzadeh A., P. Faridi, M. Shams-Ardakani & Y. Ghasemi. 2006. Medicinal smokes J. Ethnopharmacol. 108: 161-184. [ Links ]
156. Molares S. & A. H. Ladio. 2009. Ethnobotanical review of the Mapuche medicinal flora: Use patterns on a regional scale. J. Ethnopharmacol. 122: 251-260. [ Links ]
157. Montenegro P. de. 1702. Materia Médica Misionera. Obra no vista. Dato tomado de Quintana (1945). [ Links ]
158. Montenegro R. & S. Stephens. 2006. Indigenous health in Latin America and the Caribbean. Lancet 367: 1859- 1869. [ Links ]
159. Mshigeni K. E., M. H. H. Nkunya, V. Fupi, R. L. A. Mahunnah & E. N. Mshiu (eds.). 1991. Proceedings of an International Conference of Experts from Developing Countries on Traditional Medicinal Plants, University Press, Tanzania. [ Links ]
160. Nantel P., D. Gagnon & A. Nault. 1996. Population viability analysis of American Ginseng and Wild Leek harvested in stochastic environments. Conserv. Biol. 10: 608-621. [ Links ]
161. Noher de Halac R., M. Castro & E. Frank. 1985. Encuesta de datos sobre los recursos de Fauna y Flora de la Provincia de Córdoba. 80 pp., Gobierno de Córdoba, Secretaría Ministerio de Planeamiento y Coordinación. [ Links ]
162. Novara J. L. 2003. Catálogo de la Flora de la Puna en el noroeste argentino. Aportes botánicos de Salta, Ser. Misceláneas. 2: 1-51. [ Links ]
163. Novara L. 1984. Las utilidades de los géneros de Antófitas del nordeste del Valle de Lerma (Salta, República Argentina). Facultad de Ciencias Naturales, Universidad Nacional de Salta, Salta. [ Links ]
164. Nuñez C. O. & J. J. Cantero. 2000. Las plantas medicinales del Sur de la provincia de Córdoba, Fundación Universidad Nacional Río Cuarto, Córdoba, Argentina. [ Links ]
165. O'Connor B. B. 1998. Healing practices, en S. Loue (ed.), Handbook of immigrant health, pp 145-162. Plenum Press, New York. [ Links ]
166. Okuda T. 2005. Systematics and health effects of chemically distinct tannins in medicinal plants. Phytochemistry 66: 2012-2031. [ Links ]
167. Orfila E. & C. O. D'Alfonso. 1998. Catálogo preliminar de la flora medicinal serrana de Azul (Provincia de Buenos Aires, Rep. Arg.). Dominguezia 15: 27-38. [ Links ]
168. Packer L. 2004. Herbal medicine: molecular basis in health and disease management. Marcel Dekker, Inc., New York. [ Links ]
169. Palevitch P.D. & Z. Yaniv. 1991. Medicinal plants of Halyland. Vol. 1-2. Tammuz, Tel-Aviv, Israel. [ Links ]
170. Paruelo J., E. Jobbagy & O. E. Sala. 2001. Current distribution of ecosystem functional types in Temperate South America. Ecosystems 4: 683-698. [ Links ]
171. Pengelly A. 2004. The constituents of medicinal plants: an introduction to the chemistry and therapeutics of herbal medicine. CABI Publishing. Wallingford, UK. [ Links ]
172. Peña-Chacarro, M., J. De Egea, M. Jera, H. Maturo & S. Knapp. 2006. Guía de Árboles y Arbustos del Chaco Húmedo. The Natural History Museum, Guyra Paraguay, Fundación M. Bertoni y Fundación Hábitat y Desarrollo, Asunción, Paraguay. [ Links ]
173. Perez de Nucci A. 1988. La medicina tradicional del noroeste argentino. Historia y presente. Ediciones del Sol, Buenos Aires [ Links ]
174. Pérez de Paz P. & C. E. Hernández Padrón. 1999. Plantas Medicinales o útiles en la Flora Canaria. Aplicaciones populares. Francisco Lemus Editor, S. L., La Laguna, España. [ Links ]
175. Pfab M. F. & M. A. Scholes. 2004. Is the collection of Aloe peglerae from the wild sustainable? An evaluation using stochastic population modelling. Biol. Conserv. 118: 695-701. [ Links ]
176. Pieters L. & A. Vlietinck. 2005. Bioguided isolation of pharmacologically active plant components, still a valuable strategy for the finding of new lead compounds?. J. Ethnopharmacol. 100: 57-60. [ Links ]
177. Pin A. & G. Céspedes (eds.). 2009. Plantas Medicinales del Jardín Botánico de Asunción. Asociación Etnobotánica Paraguaya, Asunción. [ Links ]
178. Plotkin M. J. & L. Famolare. 1992. Conclusions and recommendations, en M. J. Plotkin & L. Famolare (eds.). Sustainable Harvest and Marketing of Rain Forest Products, pp. 310-313. Island Press, Washington, DC. [ Links ]
179. Pochettino M. & M. R. Martínez. 1998. Aporte al conocimiento actual de las plantas medicinales en Argentina: estudio etnobotánico en el Dpto. Molinos, provincia Salta, Argentina, en A. Amat (coord.). Farmcobotánica y Farmacognosia en Argentina 1980-1998, pp. 55-86. . Ediciones Científicas Americanas, La Plata. [ Links ]
180. Pochettino M. L., M. R. Martínez, B. Itten & M. Zucaro. 1997. Las plantas medicinales como recurso terapéutico en una población urbana: estudio etnobotánico en Hernández (Pdo. La Plata, Prov. Buenos Aires, Argentina) Parodiana 10: 141-152. [ Links ]
181. Principe P. P. 1989. Economics and Medicinal Plants. University of Pennsylvania Press, Philadelphia, USA. [ Links ]
182. Quintana R. 1945. Nota preliminar, en P. de. Montenegro, Materia Médica Misionera (reimpresión): V-XLVIII. Imprenta de la Biblioteca Nacional, Buenos Aires [ Links ]
183. Rabinowitz D., 1981. Seven forms of rarity, en H. Synge (ed.). The biological aspects of rare plant conservation, pp. 205-217. John Wiley & Sons, Chichester, UK. [ Links ]
184. Ramawat K. 2008. Herbal drugs: ethnomedicine to modern medicine. Springer. Berlin-Heidelberg. [ Links ]
185. Ramawat K. & J. Merillo. 2008. Bioactive molecules and medicinal plants. Springer. Berlin-Heidelberg. [ Links ]
186. Raskin I., D. Ribnicky, S. Komarnytsky, N. Ilic, A. Poulev, N. Borisjuk, A. Brinker, D. Moreno, Ch. Ripoll, N. Yakoby, J. O'Neal, T. Cornwell, I. Pastor & B. Fridlender. 2002. Plants and human health in the twenty-first century. Trends Biotechnol. 20: 522-531. [ Links ]
187. Ratera E. L. & M. O. Ratera. 1980. Plantas de la flora argentina empleadas en medicina popular. Hemisferio Sur, Buenos Aires. [ Links ]
188. Rates S. M. K. 2001. Plants as source of drugs. Toxicon 39: 603-613. [ Links ]
189. Roddick J. G. 1991. The importance of the Solanaceae in medicine and drug therapy, en J. G. Hawkes, R. N. Lester, M. Nee & N. Estrada-R (eds.). Solanaceae III. Taxonomy, Chemistry and Evolution, pp. 7-23. Royal Botanic Gardens, Kew and Linnean Society of London. [ Links ]
190. Roig F. A. 2002. Flora medicinal mendocina. Las plantas medicinales y aromáticas de la provincia de Mendoza (Argentina). Universidad Nacional de Cuyo, Mendoza. [ Links ]
191. Rondina R., A. Bandoni & J. Coussio. 2003. Plantas silvestres argentinas con reconocidas propiedades medicinales o tóxicas. OEA-CYTED, Buenos Aires. Versión en CD-ROM. [ Links ]
192. Ross I. A. 2005. Medicinal plants of the World. Chemical constituents, traditional and modern medicinal uses. Vol. 3. Human Press. New Jersey, USA. [ Links ]
193. Roth I. & H. Lindorf (eds.). 2002. South American Medicinal Plants-Botany, Remedial Properties and General Use. Springer, Berlin. [ Links ]
194. Sanz-Biset J., J. Campos-de-la-Cruz, M. Epiquién-Rivera & S. Cañigueral. 2009. A first survey on the medicinal plants of the Chazuta valley (Peruvian Amazon). J. Ethnopharmacol. 122: 333-362. [ Links ]
195. Scarpa G. 2002. Plantas empleadas contra trastornos digestivos en la medicina tradicional criolla del Chaco noroccidental. Dominguezia 18: 36-50. [ Links ]
196. Scarpa G. 2004. Medicinal plants used by the criollos of northwestern Argentine Chaco. J. Ethnopharm. 91: 115-135. [ Links ]
197. Schippmann U., D. J. Leaman & A. B. Cunningham. 2002. Impact of cultivation and gathering of medicinal plants on biodiversity: global trends and issues. Inter-Department Working Group on Biology Diversity for Food and Agriculture, FAO, Rome. [ Links ]
198. Schippmann U., D. Leaman & A. B. Cunningham. 2006. A comparison of cultivation and wild collection of medicinal and aromatic plants under sustainability aspects, en R. J. Bogers, L. E. Craker & D. Lange (eds.). Medicinal and Aromatic Plants, pp. 75-95. Springer, the Netherlands. [ Links ]
199. Schlage C., C. Mabula, R. Mahunnah & M. Heinrich. 2000. Medicinal plants of the Washambaa (Tanzania): documentation and ethnopharmacological evaluation. Pl. Biol. 2: 83-92. [ Links ]
200. Schultes R. E. & R. F. Raffauf. 1990. The Healing Forest: Medicinal and Toxic Plants of the Northwest Amazonia. Dioscorides Press, Portland. [ Links ]
201. Science Reference Services. 2008. Library of Congress. http://www.loc.gov/rr/scitech/ [ Links ]
202. Shanley P. & L. Luz. 2003. The impacts of forest degradation on medicinal plant use and implications for health care in eastern Amazonia. Bioscience 53: 573-584. [ Links ]
203. Shasany A., K. Shukla & S. P. S. Khanuja. 2007. Medicinal and aromatic plants, en C. Kole (ed.). Genome mapping and molecular breeding in plants. Plant Biotechnol. Rep. 2, pp. 93-112. Springer-Verlag, Berlin-Heidelberg . [ Links ]
204. Sheldon J. W., M. J. Balick & S. A. Laird. 1997. Medicinal plants: can utilization and conservation coexist? Advances Econ. Bot. 12: 1-104. [ Links ]
205. Shengji P. 2001. Ethnobotanical approaches of TM studies: some experiences from Asia. Pharm. Bot. 39: 74-79. [ Links ]
206. Shengji P. 2002a. A brief review of ethnobotany and its curriculum development in China, en Z. K. Shinwari, A. Hamilton, & A. A. Khan (eds.). Proceedings Workshop on Curriculum development in applied ethnobotany, pp. 21-33. Nathiagali. WWF-Pakistan, Lahore. [ Links ]
207. Shengji P. 2002b. Ethnobotany and modernisation of Traditional Chinese Medicine, en Y. A. Thomas, M. Karki, K. Gurung & D. Parajuli (eds.). Proceeding of Himalayan Medicinal and Aromatic Plants, Balancing Use and Conservation, pp. 70-78. Government of Nepal, IDRC, WWF, People and Plants, Nepal. [ Links ]
208. Shiva V. 1996. Protecting our biological and intellectual heritage in the age of biopiracy. The Research Foundation for Science, Technology and Natural Resources Policy, New Delhi. [ Links ]
209. Singh R. & C. Padmalatha. 2004. Ethno-entomological practices in Tirunelveli district, Tamil Nadu. Indian J. Traditional Knowledge 3: 442-446. [ Links ]
210. Soejarto D. D. & N. R. Farnsworth. 1989. Tropical rainforests: potential source of new drugs? Perspectives Biol. & Med. 32: 244-256. [ Links ]
211. Soejarto D. D., H. Fong, G. Tan, H. Zhang, C. Ma, S. Franzblau, C. Gyllenhaal, M. Riley, M. Kadushin, J. Pezzuto, L. Xuand, N. Hiep, N. Hung, B.Vu, P. Locd, L. Dacd, L. Binh, N. Chien, N. Hai, T. Bich, N. Cuonge, B. Southavong, K. Sydara, S. Bouamanivong, T. Van Thuyh, W. Rose & G. Dietzman. 2005. Ethnobotany/ethnopharmacology and mass bioprospecting: Issues on intellectual property and benefit-sharing. J. Ethnopharmacol.100: 15-22. [ Links ]
212. Sorarú S. B. & A. L. Bandoni. 1978. Plantas de la medicina popular. Ed. Albatros, Buenos Aires. [ Links ]
213. Struhsaker T. T. 1998. A biologist's perspective on the role of sustainable harvest in conservation. Conserv. Biol. 12: 930-932. [ Links ]
214. Svarstad H. & S. S. Dhillion. 2000. Responding to Bioprospecting: Rejection or regulation?, en H. Svarstad & S. S. Dhillion (eds.). Responding to Bioprospecting: from biodiversity in the south to medicines in the north. Spartacus Forlag AS, Oslo. [ Links ]
215. Tabuti J. R. S., S. S. Dhillion & K. A. Lye. 2003. TM in Bulamogi county, Uganda: its practitioners, users and viability. J. Ethnopharmacol. 85: 119-129. [ Links ]
216. Taylor R. S., N. P. Manandhar & G. H. Towers. 1995. Screening of selected medicinal plants of Nepal for antimicrobial activities. J. Ethnopharmacol. 46: 153-159. [ Links ]
217. Teklehaymanot T. & M. Giday. 2007. Ethnobotanical study of medicinal plants used by people in Zegie Peninsula, Northwestern Ethiopia. J. Ethnobiol. Ethnomed. 3: 12 doi:10.1186/1746-4269-3-12. [ Links ]
218. Tene V., O. Malagón, P. Vita Finzi, G. Vidari, Ch. Armijos & T. Zaragoza. 2007. An ethnobotanical survey of medicinal plants used in Loja and Zamora-Chinchipe, Ecuador. J. Ethnopharmacol. 111: 63-81. [ Links ]
219. Ticktina T. & S. Paule Dalleb. 2005. Medicinal plant use in the practice of midwifery in rural Honduras. J. Ethnopharmacol. 96: 233-248. [ Links ]
220. Toledo V. M. 1995. New paradigms for a new ethnobotany: reflections on the case of Mexico, en R. E. Schultes & S. von Reis (eds.). Ethnobotany: evolution of a discipline, pp. 75-88. Chapman and Hall, London. [ Links ]
221. Toursarkissian M. 1980. Plantas medicinales de la Argentina. Hemisferio Sur. Buenos Aires. [ Links ]
222. Tran V.O., D. Quyen, L. D. Bich, W. Jones, J. Wunder & J. Russell-Smith. 2001. A survey of medicinal plants in Ba Vi National Park, Viet Nam: methodology and implications for conservation and sustainable use. Biol. Conserv. 97: 295-304. [ Links ]
223. Tyler V. E. 1999. Phytomedicines: back to the future. J. Nat. Prod. 62: 1589-1592. [ Links ]
224. Upadhyay P., S. Roy & A. Kumar. 2007. Traditional uses of medicinal plants among the rural communities of Churu district in the Thar Desert, India. J. Ethnopharmacol. 113:387-399. [ Links ]
225. Van Wyk B. & M. Wink. 2004. Medicinal plants of the world. Briza Publications, Pretoria. [ Links ]
226. Verpoorte R. 2007. Bioprospectin: exploring our only renewable natural resorce. XXVII Annual Meeting on Micromolecular Evolution, Systematics and Ecology Reflections on the Current Status of Chemosystematics. BPL-3. São Paulo, Brasil. [ Links ]
227. Vignale N. D. 1996. Plantas medicinales del área andina de la Prov. Jujuy. Anales Saipa 14: 177-180. [ Links ]
228. Vignale N. D. 1998. Los estudios etnobotánicos en el NOA, en A. Amat (coord.). Farmacobotánica y Farmacognosia en Argentina 1980 -1998, pp. 19-54. Ediciones Científicas Americanas, La Plata. [ Links ]
229. Villamil C. 2006. La lista roja de plantas argentinas: Una herramienta para los jardines botánicos, en M. Sánchez (comp.). Plan de Acción de la Red Argentina de Jardines Botánicos, pp. 36-39. Red Argentina de Jardines Botánicos, Buenos Aires. [ Links ]
230. Villamil, C. 2004. Conservation of medicinal plants in the southern cone of South America. Med. Pl. Conserv. 9/10: 12-14. [ Links ]
231. W3 TROPICOS. 2008. Missouri Botanical Garden, St. Louis. http://www.tropicos.org/Home.aspx [ Links ]
232. Wagner H. 1977. Pharmaceutical and economic uses of the Compositae, en V. H. Heywood, J. B. Harborne & B. L. Turner (eds.). The Biology and Chemistry of the Compositae I, pp. 412-433. Academic Press INC, London. [ Links ]
233. Watanabe T., K. K. Rajbhandari, K. J. Malla & S. Yahara. 2005. A Handbook of Medicinal Plants of Nepal. Kobfa Publishing Project, Bangkok, Thailand. [ Links ]
234. Waterman P. G. 1994. Costs and benefits of secondary metabolites to the Leguminose, en J. I. Sprent & D. McKey (eds.). Advances in Legume Systematics 5: The Nitrogen Factor, pp. 129-149. Royal Botanic Gardens, Kew. [ Links ]
235. WHO. World Health Organization: TM strategy 2002- 2005. 2002 http://whqlibdoc.who.int/hq/2002/ WHO_EDM_TRM_2002.1.pdf. [ Links ]
236. Wiart Ch. 2006. Medicinal plants of Asia and the Pacific. CRC Press Inc., Boca Ratón. [ Links ]
237. Xiao P. G. & P. Yong. 1998. Ethnopharmacology and research on medicinal plants in China, en H. D. V. Prendergast, N. L. Etkin, D. R. Harris & P. J. Houghton (eds.). Plants for food and medicine, pp. 31-39. Proc. Joint Conference of the Society for Economic Botany and the International Society for Ethnopharmacology, Royal Botanic Gardens, Kew. [ Links ]
238. Xifreda C. C. 1992. Plantas útiles de la flora de la Provincia de Buenos Aires. Com. Invest. Ci. Prov. Buenos Aires, Serie Situación Ambiental, A. Recursos y rasgos naturales en la evaluación ambiental 2: 1-65. [ Links ]
239. Yaniv Z. & U. Bachrach (eds). 2005. Handbook of Medicinal Plants. Harworth Press, Inc. New York, London, Oxford. [ Links ]
240. Zamudio T. 2005. Derecho de los pueblos indígenas. http://www.indigenas.bioetica.org/index.htm, accessed 2009/03/10. [ Links ]
241. Zhang L. & A. Demain (eds.). 2005. Natural Products: Drug Discovery and Therapeutic Medicine, Humana Press. New Jersey, USA. [ Links ]
242. Zuloaga F. O., O. Morrone & M. J. Belgrano (eds.). 2008. Catálogo de Plantas Vasculares del Cono Sur (Argentina, Sur de Brasil, Chile, Paraguay y Uruguay). Vol. 1-3. Missouri Botanical Garden, Saint Louis, Missouri. [ Links ]
243. Zuloaga F. O., O. Morrone & D. Rodríguez. 1999. Análisis de la biodiversidad en plantas vasculares de la Argentina. Kurtziana 27: 17-167. [ Links ]
244. Zygadlo J. A. & H. R. Juliani. 2003. Study of essential oil composition of aromatic plants from Argentina, en D. K. Majundar, J. N. Govil & V. K. Singh (eds.). Recent progress in medicinal plants 8, pp. 273-293. Studium Press, Houston, TX. [ Links ]
Editor responsable: Leonardo Galetto.














