Introduction
Dermatophytes (DMT) are causal agents of dermatophytosis, the most important cutaneous mycosis in terms of morbidity. They are filamentous fungi parasitic on keratinized tissues that comprise three anamorphic genera: Trichophyton, Microsporum, and Epidermophyton. They are of subacute or chronic evolution, deep invasion is exceptional (1, 2, 3, 4). According to their natural habitat, they are classified as anthropophiles, zoophiles and geophiles.
Anthropophiles, such as T. rubrum, T. interdigitale, T. tonsurans, and E. floccosum, whose reservoir is human, are transmitted from one human to another. Zoophiles, such as M. canis, T. mentagrophytes, and T. verrucosum, can also affect human. Geophilic species, such as Nannizziagypsea, are found in the soil on keratin debris detached from animals and people, and can sporadically parasitize human (3, 5). These fungal species are not isolated with the same frequency in microbiology laboratories, since there is climatic, geographical, socioeconomic variability, etc., which generate changes in the distribution of these microorganisms. Although their distribution is universal, they prevail in areas with hot and humid climates (3, 6, 7).
Among the factors that predispose to dermatophytosis, we can mention high environmental temperatures, humidity, poor hygiene habits, overcrowding, use of community showers, use of closed shoes, synthetic clothing and certain underlying diseases such as: diabetes, Cushing's syndrome, lymphomas, patients infected by the human immunodeficiency virus and immunocompromised in general (6, 8).
Antifungal therapy for dermatophytosis can be topical, systemic, or a combination of both, depending on the location, extent, and severity of the lesions, the age of the patient, and the species of dermatophyte involved in the infection (9). Its use must be indicated, assessing the benefits against the possible effects (10). These drugs can be of natural or synthetic origin and have the ability to prevent the growth or cause the death of the fungus. Its mechanism of action depends on the target site on the fungal cell. They can be used topically or systemically, such as griseofulvin; azole antifungals (ATF) such as clotrimazole, itraconazole or fluconazole; allylamines such as terbinafine and new antifungal molecules (3, 7, 10). Although various ATF are available, the treatment of dermatophytoses is generally long and tedious, and some drugs are not very effective in achieving complete elimination of the infection, with the appearance of recurrences.
The treatment of onychomycosis in particular is a complex therapeutic challenge that requires prolonged, expensive antifungal therapies with numerous side effects (6, 11, 12).
In research carried out to determine the sensitivity of DMT to azoles, resistance was demonstrated in about 20% of the strains studied. However, few sensitivity studies have been published worldwide, which could be due in part to the difficulty of DMT sensitivity testing, where variables such as suitable culture medium, incubation time and temperature for conidial production, among others, must be taken into account (13, 14, 15).
Resistance to antifungal agents has stimulated the development of new and diverse compounds with a broader spectrum of antifungal action and lower toxicity than current molecules, and products of natural origin are very well received due to the extensive source of secondary metabolites with biological activity (16). Ilex paraguariensis is a plant that grows mainly in Argentina and other South American countries whose leaves and stems are processed into yerba mate, which is consumed as a popular infusion in these regions.
Studies indicate that aqueous extracts of I. paraguariensis leaves have antibacterial activity (17, 18) and on some fungal species (19). Its chemical composition has been and continues to be the subject of study, due to interest in the pharmacological properties of the compounds it possesses. Recent research has broadened the spectrum of use of yerba mate as an antimicrobial agent, crop protection and action against foodborne pathogens; its important biological activities have been attributed mainly to phenolic compounds (20, 21, 22).
The aim of this work was to determine the in vitro antifungal activity of aqueous extracts of I. paraguariensis fruits on dermatophyte fungi isolated from clinical samples.
Materials and methods
Strains of dermatophyte fungi isolated from clinical samples of patients from the area of Gran Posadas, referred to the mycological diagnostic service "Fungal Isolations of Medical Interest" of the Faculty of Exact, Chemical and Natural Sciences (National University of Misiones), during a period of 4 years, were used.
The study was descriptive cross-sectional, with non-probability convenience sampling. Fungal isolates were identified by classical mycological methods, which included direct microscopic observation of clinical material for fungal elements; cultures in a battery of culture media that ensured fungal isolation, including: Sabouraud dextrose 4% agar (Merck-Merck Química Argentina), fungal and yeast agar (Britania), selective agar for pathogenic fungi (Merck-Merck Química Argentina).
Cultures were incubated at 28 °C and fungal species were identified based on the macroscopic, microscopic and physiological morphological characteristics of the cultures developed. The isolated strains were kept in water agar (AA) and oatmeal agar (AHA) refrigerated at 4-8 ºC (6, 23).
The aqueous extract of yerba mate fruits (EAFYM) was prepared with mixed green and red fruits, using the decoction method (24), and subjected to freeze-drying for preservation. For ATF sensitivity activity tests, the microdilution method was used according to CLSI document M38, 3rd ed. (25).
Terbinafine (TERBI) and itraconazole (ITRAC), antifungals with very good activity against dermatophytes (26, 27, 28), were used as controls. In order to assess the antifungal activity (AATF) of JMEA and since no standardised methods exist, modifications to the CLSI document were implemented. From the dry plant extract, a stock solution of 500 mg/ml was prepared in sterile distilled water and total polyphenols were determined and a value of 0.54 g EAG/100 ml was obtained by the Folin-Ciocalteau method. Stock solution was diluted 1:2 in RPMI1640 medium so that the highest concentration was 250 mg/ml and the lowest was 0.49 mg/ml; 100 μl of the AGE dilutions were then dispensed into columns 1 to 10 of the culture microplates, with columns 11 and 12 containing RPMI, for growth control and sterility, respectively.
To obtain the inocula to be used, the isolates were subcultured on AHA medium for 7 days at 28 °C. The colonies obtained were covered with 1-2 ml of sterile physiological solution, scraping the surface with a loop to obtain suspensions of the aerial mycelium of the colonies.
The fungal suspension of conidia and hyphae thus obtained was transferred to a sterile conical tube, allowing it to stand for 10 min to allow the precipitation of heavier particles. The supernatant was transferred to another sterile tube and vortexed (15 seconds). The fungal inoculum was adjusted by counting the conidia in a Neubauer chamber.
The conidia suspension had twice the concentration of the final concentration for the test (1-3x103 CFU/ml). 100 µl per well of the microdilution plate were inoculated except in column 12, and incubated at 35ºC, without shaking, inside a plastic box with moistened filter paper to prevent dehydration of the medium. Readings were made visually at 24, 48, 72 and 96 h. The minimum inhibitory concentration (MIC) reading point was defined as the lowest concentration that inhibited discernible growth (100% inhibition).
Results
We worked with 50 pure strains of dermatophytes from different anatomical sites (hairless skin, hair and nails), 12 strains of T. mentagrophytes, 13 strains of T. rubrum, 16 strains of M. canis, 6 strains of N. gypsea and 3 strains of T. tonsurans.
The MIC of the commercial ATF used as controls can be seen in Table N°1. In the case of TERBI, they were 0.03-0.50 µg/ml and for ITRAC, 0.12-4 µg/ml.
Table1: Number of DMT strains and cumulative % inhibition against ATF controls.
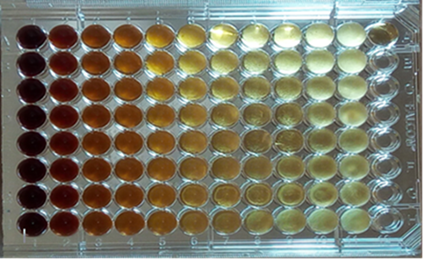
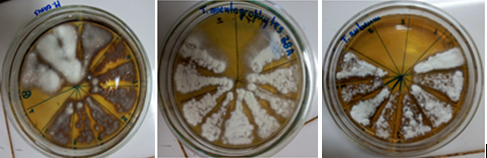
To determine the MIC of EAFYM against DMT and due to the dark amber color of the extract that made it difficult to visualize fungal growth at the highest concentrations (Figure Nº1), the contents of wells 1 to 11 were repicked in Sabouraud dextrose agar 2%. In this way, the inhibitory concentration of fungal development could be accurately established (Figure N°2).
Figure 1: MIC of EAFYM versus DMT. Culture plate with 96 h of incubation.
Wells 1 to 10: EAFYM dilutions (125 to 0.24 mg/ml)
Well 11: Fungal Growth Control
Well A-12: RPMI Sterility control
Figures2: Replicates for MIC confirmation of EAFYM against different strains of DMT.
The MIC of the EAFYM were in a range of 31.25 to 125 mg/ml as can be seen in Table N°2.
Table 2: Number of DMT strains and cumulative % inhibition against EAFYM.
Discussion
Dermatophytoses are a major problem in the world population of all ages (1, 3, 6, 29). The azoles and allylamines are considered effective in the antifungal therapy of these mycoses; however, in the case of onychomycosis, ATF treatment is complex and often unsatisfactory; it requires long, costly treatments with numerous side effects (5,11, 30, 31).
Unfortunately, there are insufficient publications on sensitivity studies of DMT compared to ATF commonly used in medical practice (13, 14, 32, 33). In the present study, it was observed that compared to the DMT strains studied, the most active ATF was TERBI, which presented MIC between 0.03 and 0.50 µg/ml and ITRAC values between 0.12 and 4 µg/ml; results similar to those reported by Serrano-Martino (13), Perez-Cardenas et al. (26), DiazJarabrán et al. (27), Santos et al. (33) and Manzano Gayosso et al. (3. 4).
In the search for new compounds with antifungal action and lower toxicity than current ones, products of natural origin, a huge source of secondary metabolites with biological activity, have been very well received (16, 35, 36).Numerous publications report on the biomedical and pharmacological properties of I. paraguariensis(37, 38, 39) but few on the possibility of its use as an antimicrobial (17, 20, 40).On its fruits, which constitute a residue in the yerba mate industry, there are very few scientific studies carried out regarding its phytochemical and/or pharmacological properties (22); and practically null on their antimicrobial capacity.The evaluation of plant extracts on the inhibition of microorganisms at the in vitro level is the first phase that indicates the promising potential of a plant to be used as a natural antimicrobial.
As there is no standardized technique for the study of plant extracts by the broth dilution method, we have implemented modifications to what is proposed in the CLSI document M38, 3rd ed.; it was started from a stock solution of EAFYM of 500 mg/ml and the AATF was evaluated in a range of 250 - 0.49 mg/ml. However, by this method visualization of fungal growth was difficult at the highest concentrations of the extract, due to the dark amber coloration of the EAFYM. To sort out the difficulty presented in the reading of the MIC of the EAFYM, microscopic observations and peals of the cultures in the different concentrations of the EAFYM were made to accurately establish the minimum concentration that inhibited fungal development.
Other researchers (41, 42), in studies to determine the AATF of plant extracts, worked with modifications of the broth microdilution method proposed by the CLSI, and included resazurin as a colorimetric indicator with very good results.
The results of this study allowed to demonstrate the in vitro AATF of the aqueous extract of the fruits of I.paraguariensis. The MIC of the EAFYM that inhibited the fungal development of the DMT strains studied, presented values between 31.25 and 125 mg/ml. No publications on the antifungal activity of the fruits of I.paraguariensis were found. However, there are studies that have managed to demonstrate the antifungal action of aqueous extracts from I. paraguariensis leaves. Filip et al. (43) found inhibitory activity on Malassezia furfur at a concentration of 1000 mg/ml; Medvedeff et al. (19), observed 80% growth inhibition of the fungal species Fusarium oxysporum, at a concentration of 60 mg/ml of plant extract. Inhibitory concentrations comparable to those found in the present study. However, Burris (20), published very good antimicrobial activity of I. paraguariensis against E. coli and S. aureus and determined that the minimum bactericidal concentrations were approximately 150 to 800 μg/ml and 25 to 50 μg/ml, respectively. Ruiz Quiroz (42) demonstrated that the ethanolic and hydroalcoholic extracts of Ilex guayusa leaves have inhibitory activity against strains of Candida spp. and M. canis at concentrations ranging from 62.5 to 250 μg/ml.
Several authors have studied the relationship between polyphenol content and antimicrobial activity, and found an association between them in different types of extracts from I. paraguariensis leaves; leaf and fruit extracts of other Ilex species and other plants (20, 21, 44, 45). Haraguchi et al. (46) attributed the antimicrobial action of the Ilex genus to triterpenes, especially rotundic acid. Taking into account this association, and that in the present study, the concentration of total phenols measured in the aqueous extracts of I. paraguariensis fruits was lower than that reported by other authors in plant extracts with good antimicrobial activity. It is considered appropriate to use, in future studies, other extractive methods, as well as the identification and separation of the active components of the fruits of yerba mate, to improve the antifungal capacity found in this work. The AATF of the fruits of I. paraguariensis, demonstrated in the present work, added to the results obtained by other authors in trials that demonstrated the absence of acute toxic effects in experimental animals (22), allow these fruits to be considered, with the possibility of being used in the preparation of natural antifungal medicines.
Conclusions
The results obtained under the test conditions used lead to the conclusion that EAFYM has in vitro antifungal activity against strains of T. mentagrophytes, T. rubrum, M. canis, N. gypsea and T. tonsurans, with MIC values ranging from 31.25 to 125 mg/ml, concentrations much higher than TERBI and ITRAC.
The antifungal property of EAFYM found in the present work stimulates to continue the study with the application of other extractive methods, as well as the identification and separation of the active components of the fruit of I. paraguariensis that could be positioned as a natural antifungal.
Received: 28/12/2021
Accepted: 10/11/2022